Abstract
Review Article
Use of MicroRNAs to Screen for Colon Cancer
Farid E Ahmed*, Nancy C Ahmed, Mostafa Gouda and Chris Bonnerup
Published: 31 August, 2017 | Volume 1 - Issue 1 | Pages: 045-074
Colon cancer (CC) screening is important for diagnosing early stage for malignancy and therefore potentially reduces mortality from this disease because the cancer could be cured at the early disease stage. Early detection is needed if accurate and cost effective diagnostic methods are available. Mortality from colon cancer is theoretically preventable through screening. The Current screening method, the immunological fecal occult blood test, FOBTi, lacks sensitivity and requires dietary restriction, which impedes compliance. Moreover colonoscopy is invasive and costly, which decreases compliance, and in certain cases could lead to mortality. Compared to the FOBT test, a noninvasive sensitive screen that does not require dietary restriction would be more convenient. Colonoscopy screening is recommended for colorectal cancer (CRC). Although it is a reliable screening method, colonoscopy is an invasive test, often accompanied by abdominal pain, has potential complications and has high cost, which have hampered its application worldwide.
A screening approach that uses the relatively stable and nondegradable microRNA molecules when extracted from either the noninvasive human stool, or the semi-invasive blood samples by available commercial kits and manipulated thereafter, would be more preferable than a transcriptomic messenger (m)RNA-, a mutation DNA-, an epigenetic-or a proteomic-based test. That approach utilizes reverse transcriptase (RT), followed by a modified quantitative real-time polymerase chain reaction (qPCR). To compensate for exosomal miRNAs that would not be measured, a parallel test could be performed on stool or plasma’s total RNAs, and corrections for exosomal loss are made to obtain accurate results. Ultimately, a chip would be developed to facilitate diagnosis, as has been carried out for the quantification of genetically modified organisms (GMOs) in foods. The gold standard to which the miRNA test is compared to is colonoscopy. If laboratory performance criteria are met, a miRNA test in human stool or blood samples based on high throughput automated technologies and quantitative expression measurements currently employed in the diagnostic clinical laboratory, would eventually be advanced to the clinical setting, making a noticeable impact on the prevention of colon cancer.
Read Full Article HTML DOI: 10.29328/journal.hjbm.1001006 Cite this Article Read Full Article PDF
Keywords:
Bioinformatics; Diagnosis; Histopathology; Microarrays; QC, RNA, RT-qPCR, Statistics
References
- Peterson NB, Murff HJ, Ness RM, Dittus RS. Colorectal cancer screening among men and women in the United States. J Womens Health. 2007; 16: 57-65. Ref.: https://goo.gl/3EDoae
- Mandel JS. Screening for colorectal cancer. Gastrointestinal Clin N Ame. 2008; 37: 97-115. Ref.: https://goo.gl/oCuYge
- Davies RJ, Miller R, Coleman N. Colorectal cancer screening: prospects for molecular stool analysis. Nature Rev Cancer. 2005; 5: 199-209. Ref.: https://goo.gl/Pg1myj
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2009. A review of current American Cancer Society Guidelines and issues in cancer screening. CA Cancer J Clin. 2009; 59: 27-41. Ref.: https://goo.gl/KcMdMa
- Centers for Disease Control and Prevention. Increased use of colorectal cancer test: United States, 2002 and 2004,MMWR Mortal Wkly. 2006; 55: 208-311. Ref.: https://goo.gl/dtF1qt
- Ahmed FE. Colon cancer: Prevalence, screening, gene expression and mutation, and risk factors and assessment. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003; 21: 65-131. Ref.: https://goo.gl/s1THQB
- Morikawa T, Kato J, Yamaji Y, Wada R, Mitsushima T, et al. A Comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129: 422-428. Ref.: https://goo.gl/qFWRqy
- Kohler BA, Ward E, McCarthy BJ, Edwards BK, Jemal A, et al. Annual report to the nation on the status of cancer, 1975-2007, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010; 116: 544-573. Ref.: https://goo.gl/HEU4v3
- Ahlquist DA. Fecal occult blood testing for colorectal cancer. Can we afford to do this? Gastroenterol Clin N Amer. 1997; 26: 41-55, Ref.: https://goo.gl/CiDZiY
- Davidson LA, Lupton JR, Miskovsky E, Miskovsky, Alan P. Fields, et al. Quantification of human intestinal gene expression profiling using exfoliated colonocytes: a pilot study. Biomarkers. 2003; 8: 51-61. Ref.: https://goo.gl/kam5TN
- Ahmed FE, Jeffries CD, Vos PW, Flake G, Nuovo GJ, et al. Diagnostic microRNA markers for screening sporadic human colon cancer and ulcerative colitis in stool and tissue. Cancer Genom Proteom. 2009; 6: 281-296. Ref.: https://goo.gl/7cKJBE
- Ahmed FE, Vos P, iJames S, Lysle DT, Allison RR, et al. Transcriptomic molecular markers for screening human colon cancer in stool & tissue. Cancer Genom Proteom. 2007; 4: 1-20, 2007. Ref.: https://goo.gl/7yJ3eB
- Ahmed FE, Ahmed NC, Vos PW, Bonnerup C, Atkins JN,et al. Diagnostic microRNA markers to screen for sporadic human colon cancer in stool: I. Proof of principle. Cancer Genom Proteom. 2013; 10:93-113. Ref.: https://goo.gl/FPuKfyc
- Ahmed FE, Ahmed NC, Vos PW, Bonnerup C, Atkins JN, et al. Diagnostic microRNA markers to screen for sporadic human colon cancer in blood. Cancer Genom Proteom. 2012; 9: 179-192. Ref.: https://goo.gl/EDntph
- Ahlquist DA. Fecal occult blood testing for colorectal cancer. Can we afford to do this? Gastroenterol Clin North Am. 1997; 26: 41-55. Ref.: https://goo.gl/htMCvJ
- Cheng L, Eng G, Nieman L, Kapadia AS, Du XL.Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol. 2011; 34: 573-580. Ref.: https://goo.gl/VkxgTB
- Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, et al.The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. J Natl Cancer Inst. 2009; 125: 171-180. Ref.: https://goo.gl/i4eq6N
- Morikawa T, Kato J, Yamaji, Wada R, Mitsushima T, et al. Comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology. 2005; 129: 422-428. Ref.: https://goo.gl/iHrxpa
- Newcomb PA, Storer BE, Morimoto LA, Templeton A, Potter JD. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst. 2003; 95: 622-625. Ref.: https://goo.gl/iAzxbz
- Yamai Y, Mitsushima T, Ikuma H, Watabe H, Okamoto M, et al.Right-sided shift of colorectal adenomas with aging. Gastrointest Endoscopy. 2006;63: 453-458. Ref.: https://goo.gl/ZPW9Cj
- Gatto NM, Frucht H, Sundarararjan V, Jacobson JS, Grann VR, et al. Risk of perforation after colonoscopy or sigmoidoscopy: a population based study. J Natl Cancer Inst. 2003; 95: 230-236. Ref.: https://goo.gl/QzDjNM
- Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, et al. Differential gene expression in colon cancer of the ceacum versus the sigmoid and rectosigmoid. Gut. 2005; 54: 374-384. Ref.: https://goo.gl/Cv6fVD
- Gervaz P, Bouzourene H, Gerottini JP. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor locations. Dis Colon Rectum. 2001; 44: 364-372.
- Bressler B, Paszat LF, Vinden C, Li C, He J, et al. Colonoscopic miss rates for right-sided colon cancer: population-based study.Gastroenterology. 2004; 127: 452-456. Ref.: https://goo.gl/gqM49H
- Mulhall BP, Veerappan GR, Jackson J. Meta-analysis: Computed tomographic colonography. Ann Intern Med. 2005; 142: 635-650. Ref.: https://goo.gl/Fhefc9
- Kealey SM, Dodd JD, MacEneaney PM, Gibney RG, Malone DE. Minimal preparation computed tomography instead of barium enema/colonoscopy for suspected colon cancer in frail elderly patients: an outcome analysis study. Clinical Radiol. 2004; 59: 44-52. Ref.: https://goo.gl/pYJV17
- Mȕller H M, Oberwalder M, Fiegl H, Morandell M, Goebel G, et al. Methylation changes in fecal DNA: a marker for colorectal cancer screening. Lancet. 2004; 363: 1283-1285. Ref.: https://goo.gl/uJuByP
- Lenhard K, Bommer GT, Asutay S, Schauer R, Brabletz T, et al. Analysis of promoter methylation in stool: a novel method for the detection of colorectal cancer. Clin Gastroenterol Hepatol. 2005; 3: 142-149. Ref.: https://goo.gl/FJi9r5
- Itzkowitz SH, Jandorf L, Brand R, Rabeneck L, Schroy PC 3rd, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007; 5: 111-117. Ref.: https://goo.gl/yh4Ln5
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME, et al. Fecal DNA versus fecal occult blood for colorectal cancer screening in an average-risk population. New Eng J Med. 2004; 351: 2704-2714. Ref.: https://goo.gl/ivNqvJ
- Ahmed FE. Liquid chromatography-mass spectrometry: A tool for proteome analysis & biomarker discovery and validation. Exp Opin Mol Diag. 2009; 3: 429-444. Ref.: https://goo.gl/icM5wn
- Osborn NK, Ahlquist DA. Stool screening for colorectal cancer: molecular approaches. Gastroenterology. 2005;128: 192-206. Ref.: https://goo.gl/ghMA4Q
- Ahlquist DA, Shuber AP. Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clin Chim Acta. 2002; 315: 151-157. Ref.: https://goo.gl/AJkUr2
- Traverso G, Shuber A, Levin B, Johnson C, Olsson L, et al. Detection of APC mutations in fecal and DNA from patients with colorectal tumors. New Engl J Med. 2002; 346: 311-320. Ref.: https://goo.gl/o5Svd1
- Ahlquist DA, Skoletsky JE, Boynton KA, Harrington JJ, Mahoney DW, et al. Colorectal cancer screening by detection of altered human DNA in stool: feasibility of a multitarget assay panel. Gastroenterology. 2000; 119: 1219-1227. Ref.: https://goo.gl/Zh284Z
- Ladabaum U and Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastroenterology. 2005; 129: 1151-1126. Ref.: https://goo.gl/KDLhgo
- Polley AC, Mulholland F, Pin C, Williams EA, Bradburn DM, et al. Proteomic analysis reveals field-wide changes in protein expression in the morphologically normal mucosa of patients with colorectal neoplasia. Cancer Res. 2006; 66: 6553-6562. Ref.: https://goo.gl/vER2Ub
- Xin B, Platzer P, Fink SP, Reese L, Nosrati A, et al. Colon cancer secreted protein-2 (CCSP-2) a novel candidate serological marker of colon neoplasia. Oncogene. 2005; 24: 724-731. Ref.: https://goo.gl/WPSSdC
- Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008; 283, 15647-15655. Ref.: https://goo.gl/xRW845
- Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981; 212: 53-55. Ref.: https://goo.gl/X55j65
- Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of the early detection guidelines for prostate, colorectal and endometrial cancers. CA Cancer J Clin. 2001; 51: 38-75.
- Ng EKO, Chong WWS, Jin H, Lam EK, Shin VY, et al. Differential expression of microRNA in plasma of patients with colorectal cancer: A potential marker for colorectal cancer screening. Gut. 2009; 58: 1375-1381.Ref.: https://goo.gl/vdrg7u
- Link A, Balaguer F, Shen Y, Nagasaka T, Lozano JJ, et al. Fecal miRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev. 2010; 19: 1766-1774. Ref.: https://goo.gl/T8zM6n
- Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res. 2010; 3: 1435-1442. Ref.: https://goo.gl/omh9cj
- Kalimutho M, Del Vecchio BG, Di Cecilia S, Sileri P, Cretella M, et al. Differential expression of miR-144* as a novel fecal-based diagnostic marker for colorectal cancer. J Gastroenterol. 2011; 46: 1391-1402. Ref.: https://goo.gl/8ofmSf
- Kalimutho M, Di Cecilia S, Del Vecchio BG, Roviello F, Sileri P, et al. Epigenetically silenced miR-34b/c as a novel faecal-based screening marker for colorectal cancer. Br J Cancer. 2011; 24: 1770-17780. Ref.: https://goo.gl/S6BHd2
- Kunte DP, Delacruz M, Wali RK, Menon A, Du H, et al. Dysregulation of microRNAs in colonic field carcinogenesis: implications for screening. PLoS One. 2012; 7. Ref.: https://goo.gl/9Uv87v
- Wu CW, Ng SS, Dong YJ, Ng SC, Leung WW, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut. 2012; 61: 739-745. Ref.: https://goo.gl/KM4KTC
- Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA Jr, et al. The colorectal microRNome. Proc Natl Acad Sci USA. 2006; 103: 3687-3692. Ref.: https://goo.gl/1ffTMh
- Schepler T, Reinert JT, Oslenfeld MS, Christensen LL, Silahtaroglu AN, et al. Diagnostic and prognostic microRNAs in Stage II colon cancer. Cancer Res. 2008; 68: 6416-6424. Ref.: https://goo.gl/7jxmdN
- Barbarotto E, Schmittgen TD, Calin GA. MicroRNAs and cancer: Profile, profile, profile. Int J Cancer. 2008; 122: 969-977. Ref.: https://goo.gl/AAH3YA
- Schetter AJ, Leung SY, Sohn JJ, Harris HH, Calin GA, et al. MicroRNA expression profile associated with progression and therapeutic outcome in colon adenocarcinoma. J Am Med Assoc. 2008; 299: 425-436. Ref.: https://goo.gl/rG9qaK
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006; 6: 857-866. Ref.: https://goo.gl/a4VaZA
- Lu J, Getz G, Miska EA, Eric A, Alvarez-Saavedra, Ezequiel, et al. MicroRNA expression profiles classify human cancers. Nature. 2005; 435: 834-838. Ref.: https://goo.gl/2tBaAP
- Yantis RK, Goodarzi M, Zhou XK, Rennert H, Pirog EC, et al Clinical, pathological, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol. 2009; 33: 572-582. Ref.: https://goo.gl/Pkcs1N
- Luo X, Burwinke B, Tao S, Brenner J. MicroRNA signatures: Novel biomarkers for colorectal cancers. Cancer Epidemiol Biomarkers Prev. 2011; 20: 1272-1286. Ref.: https://goo.gl/rz2aXY
- Ahmed FE Testing for genetically modified organisms (GMOs) in food products. Lab Plus Intern. 2002; 16: 8-16.
- Ahmed FE, Vos P Molecular markers for human colon cancer in stool and blood identified by RT-PCR. Anticancer Res. 2004; 24: 4127-4134.
- Wang K, Zhang S. Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010; 38: 7248-7259. Ref.: https://goo.gl/7Z4k68
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, et al. Argonaute 2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011; 108: 5003-5008. Ref.: https://goo.gl/6ithpn
- Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011; 13: 423-433. Ref.: https://goo.gl/LVkXDu
- Hunter MP. Detection of microRNA expression in human peripheral blood microvessicles. PLoS One. 2008; 3: e3694. Ref.: https://goo.gl/VCsF6q
- Shaffer J, Schlumpberger M, Lader E. miRNA profiling from blood-Challenges and recommendations. 2012; 1-10. Ref.: https://goo.gl/UzZJcA
- Ahmed FE, James SI, Lysle DT, Johnke RM, Flake G, et al. Improved methods for extracting RNA from exfoliated human colonocytes in stool and RT-PCR analysis. Dig Dis Sci. 2004; 49: 1889-189. Ref.: https://goo.gl/ZL3RF1
- Mestdagh P, Van Vlierberghe P, Weer De, Muth D, Westermann F, et al. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biology. 2009; 10: R64. Ref.: https://goo.gl/NcZsbM
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005; 37: 766-770. Ref.: https://goo.gl/SdnC8n
- Balcells I, Cirera S, Busk PK. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011; doi: 10.1186/1472-6750-11-70. Ref.: https://goo.gl/acP9sf
- Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009; 112: 55-59. Ref.: https://goo.gl/r3FHeQ
- Redshaw N, Wilkes T, Whale A, Cowen S, Huggett J, et al. A comparison of miRA isolation and RT-qPCR technologies and their effects on quantification accuracy and repeatability. BioTechniques. 2013; 54: 155-164. Ref.: https://goo.gl/odZL4n
- Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, et al. Expression profiling identifies distinct microRNA signature in pancreatic cancer. Int J Cancer.2007; 120: 1046-1054. Ref.: https://goo.gl/GkjLKn
- Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006; 9: 189-198. Ref.: https://goo.gl/iaXvCV
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005; 65: 7065-7070. Ref.: https://goo.gl/gRnTYF
- Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Rakic P, et al. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 2004;5: R68. Ref.: https://goo.gl/aGnWQi
- Kim J, Krichevsky A, Grad Y, Gabriel D, Kenneth S, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci USA. 2004; 101: 360-365. Ref.: https://goo.gl/g4rd6K
- Volinia S, Calin GA, Liu CG, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA. 2006; 103: 2257-2261. Ref.: https://goo.gl/tdtGu8
- Aandrés E, Cubedo E, Agirre X, Malumbres R, Navarro A, et al. Identification by real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumor tissues. Mol Cancer. 2006;5: 29. Ref.: https://goo.gl/QsXhHD
- Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005; 33: 5394-5403. Ref.: https://goo.gl/hYNyXD
- Shi B, Stepp-Lorenzino L, Prisco M, Linsley P, Baserga R, et al. MicroRNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem. 2007; 282: 32582-32590. Ref.: https://goo.gl/fx31Av
- Calin GA, Ferracin M, Cimmino A, Shimizu M, Visone R, et al. A microRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. N Eng J Med. 2005; 353: 1793-1801. Ref.: https://goo.gl/9xaxAH
- Eis PS, Tam W, Sun L, Chadburn A, Li Z, et al. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA. 2003; 102: 3627-3632. Ref.: https://goo.gl/3tiZ6j
- Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, et al. Frequent deletions and downregulation of microRNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acas Sci USA. 2002; 99: 15524-15529. Ref.: https://goo.gl/k8rtMh
- Nybo K, Lo PCH. Optimal miRNA RT-qPCR. BioTechniques. 2013;54: 113.
- Ahmed FE, Vos PW, Clark J, Wiley JE, Weidner DA, et al. Differences in mRNA and microRNA expression profiles in human colon adenocarcinoma HT-29 cells treated with either intensity-modulated radiation therapy (IMRT), or conventional radiation therapy (RT). Cancer Genom Proteom. 2009; 6: 109-127. Ref.: https://goo.gl/PQodJ6
- Wu F, Zikusoka M, Trindade A, Dassopoulos T, Chakravarti S, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory particle 2-α. Gastroenterology. 2008; 135: 1626-1635. Ref.: https://goo.gl/edUJbv
- Lu M, Zhang Q, Deng M, Miao, Cui Q, et al. An analysis of human microRNA and disease associations. PLoS One. 2008; 3: e3420. Ref.: https://goo.gl/YQYE4V
- Ahmed FE Expression microarray proteomics and the search for cancer biomarkers. Curr Genomics. 2006; 7: 399-426. Ref.: https://goo.gl/fvhGMA
- Ahmed FE. Quantitative real-time RT-PCR: Application to carcinogenesis. Cancer Genom Proteom. 2005; 2: 317-332. Ref.: https://goo.gl/6WZE5f
- Lewis BP, Shih IH, Jones-Rhodes MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003; 115: 787-789. Ref.: https://goo.gl/EQCSzC
- Gusev Y. Computational methods for analysis of cellular functions and pathways collectively targeted by differentially expressed microRNA. Methods. 2008; 44: 61-72. Ref.: https://goo.gl/BgFKoA
- Gusev Y, Schmittgen TD, Lerner M, Postier R, Brackett D. Computational analysis of biological functions and pathways collectively targeted by coexpressed microRNAs in cancer. BMC Bioinformatics. 2007; 8(Suppl 7): S16. Ref.: https://goo.gl/4wsGK5
- Ahmed FE. The role of microRNA in carcinogenesis and biomarker selection: a methodological perspective. Exp Rev Mol Diag. 2007; 7: 569-603. Ref.: https://goo.gl/krV5s9
- Sobin LH, Wittekind CH. eds UICC TNM Classification of Malignant Tumors, 6th Edition. New York, John Wiley. 2002; 170-173.
- Greene FL, Page DL, Fleming ID. Eds AJCC Cancer Staging Manual. 6th Edition. Springer-Verlag, New York. 2002.
- DeBakey ME, Yang L, Belaguli N. MicroRNA and colorectal cancer. World J Surg. (2009); 33: 638-646. Ref.: https://goo.gl/pHHkda
- Zhou X, Ruan J, Wang G, Zhang W. Characterization and identification of microRNA core promoters in trout model species. PLoS Comput Biol. 2005; 3: e37. Ref.: https://goo.gl/QeaCdV
- Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNA using deep-sequencing data. Nucleic Acids Res. 2014; 42: D68-D73. Ref.: https://goo.gl/725cpz
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism and function. Cell. 2004;116: 281-297. Ref.: https://goo.gl/dzyaTz
- Reinhart BJ, Slack FJ, Basson M et al RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403: 901-906. Ref.: https://goo.gl/t4bGUJ
- Xu P, Guo M, Hay BA. MicroRNAs and the regulation of cell death. Trend Genet. 2004; 20: 617-624. Ref.: https://goo.gl/rek2Ao
- Chang-Zheng C. MicroRNAs as oncogenes and tumor supressors. N Eng J Med. 2005; 353: 1768- 1771. Ref.: https://goo.gl/1jXDzK
- Calin GA, Sevignai C, Dumitru CD, Hyslop T, Noch E, et al. Human microRNAs are frequently located at fragile sites and genomic regions involved in cancer. Proc Natl Acad Sci USA. 2004; 101: 2999-3004. Ref.: https://goo.gl/8S2Ut9
- Ahmed FE. Molecular markers that predict response to colon cancer therapy. Exp Rev Mol Diag. 2005; 5: 353-375. Ref.: https://www.ncbi.nlm.nih.gov/pubmed/15934813
- Lanza G, Ferracin M, Gafà R, Veronese A, Spizzo R, et al. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Molecular Cancer. 2007; 6: 54. Ref.: https://goo.gl/EKzZ3r
- Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, et al. A combined computational-experimental approach predicts human microRNA targets. Genes Dev. 2004; 18: 1165-1178. Ref.: https://goo.gl/fipMMv
- John BB, Enright AJ, Aravin A, Tuschl T, Sander C, et al. Human microRNA target. PloS Biol. 2004; 2: e363. Ref.: https://goo.gl/2mFVB9
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, et al.Combinational microRNA target predictions. Nature Genet. 2005; 37: 495-500. Ref.: https://goo.gl/4zo78L
- Stark A, Brennecke J, Bushati N, Russell RB, Cohen SM. Animal microRNAs confers robustness to gene expression and have a significant impact on 3’UTR evaluation. Cell. 2005; 123: 1133-1146. Ref.: https://goo.gl/p3Gvna
- Oberg AL, French AJ, French AJ, Subramanian S, Morlan BW et al. MiRNA expression in colon polyps provide evidence for a multihit model of colon cancer. PLoS ONE. 2011; 6: e20465. Ref.: https://goo.gl/h6uvVY
- Valadi H, Elkstrom K, Bossios A,Sjöstrand M, Lee JJ, et al. Exosome mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9: 654-659. Ref.: https://goo.gl/hPT9bm
- Ahmed FE. Laser microdissection: application to carcinogenesis. Cancer Genom. Proteom. 2006; 3: 217-226.
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inbibitors. Proc Natl Acas Sci USA. 1977; 74: 5463-5467. Ref.: https://goo.gl/dTJRbo
- Morozova O, Marra MA. Application of next-generation sequencing technologies in functional genomics. Genomics. 2008; 92: 255-264. Ref.: https://goo.gl/n7gTfz
- Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1988; 8: 186-194. Ref.: https://goo.gl/aefkk9
- Margulies M, Engholm M, Altman WE, Attiya S, Bader JS, et al. Genome sequencing in microfabricated high-density picoliter reactors. Nature. 2005; 437: 376-380. Ref.: https://goo.gl/8txWVi
- Bentley DR. Whole-genome re-sequencing. Curr Opin Genet Dev. 2006; 16: 545-552. Ref.: https://goo.gl/rnJPes
- Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, et al. Accurate multiplex polony sequencing at an evolved bacterial genome. Science. 2005; 309: 1728-1732. Ref.: https://goo.gl/QMz8kk
- Jensen SG, Lamy P, Rasmussen MH, Ostenfeld MS, Dyrskjøt L, et al. Evaluation of two commercial global miRNA expression profiling platforms for detection of less abundant miRNAs. BMC Genomics. 2011; 12: 435. Ref.: https://goo.gl/pSZgPA
- Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee DH, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005; 33: e179. Ref.: https://goo.gl/U6PHMc
- Tellman G. The E-method: a highly accurate technique for gene-expression analysis. Nature Methods. 2006; 3: 1-2.
- Light Cycler Software®, Version 3.5, Roche Molecular Biochemicals, Mannheim, Germany, 2001; 64-79.
- Luu-The V, Paquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques. 2005; 38: 287-293. Ref.: https://goo.gl/uTwGx8
- Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999; 75: 291-295. Ref.: https://goo.gl/CHj4EB
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. Accurate normalization of real-time quantitative RT-PCR by geometric average of multiple internal control genes. Genome Biol. 2002; 3. Ref.: https://goo.gl/ywZczE
- DeMuth JP, Jackson CM, Weaver DA, Erin L Crawford, Dennis S, et al. The gene expression index cmyc x E2F-1/p21 is highly predictive of malignant phenotype in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1998; 19: 18-29. Ref.: https://goo.gl/YH5yrc
- Nagan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, et al. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. 2007; 95: 652-662. Ref.: https://goo.gl/jKs2S9
- Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the occurance of a biomarker used for classification or prediction: standards for study design of Cancer. J.Natl Cancer Inst. 2008; 100: 1432-1438. Ref.: https://goo.gl/Z7zdpA
- Ein-Dor L, Zuk O, Domany E. Thousands of samples are needed to generate a robust gene list for predicting outcome in cancer. Proc Natl Acad Sci USA. 2006; 103: 5923-5928. Ref.: https://goo.gl/koxRNC
- Schwarzenbach H, da Silva A A, Calin G, Pantel K. DNA normalization strategies for microRNA quantification. Clinical Chem. 2015; 61: 1333-1342. Ref.: https://goo.gl/9LB8DZ
- Bustin SA, ed. A-Z of Quantitative PCR. International University Line, La Jolla, CA, 2004.
- Yau TO, Wu CW, Dong Y, Tang CM, Ng SS, et al. MicroRNA-221 and microRNA-18a identification in stool as biomarkers for the non-invasive diagnosis of colorectal carcinoma. Br J Cancer. 2014; 111: 1765-1771. Ref.: https://goo.gl/AUAvH2
- Tichopad A, Dilger M, Schwarz G, Pfaffl MW. Standardised determination of real-time PCR efficiency from a single reaction setup. Nucleic Acids Res. 2003; 31. Ref.: https://goo.gl/eNTPgw
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, et al. The MIQUE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009; 55: 611-622. Ref.: https://goo.gl/fhjcRN
- Cornell RG, Ed. Statistical models for cancer studies. In Models to Analyze Strategies in the General Population, 346-347, Marcel Dekker, NY, 1984.
- Sureh KP, Chandrashekare S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012; 5: 7-13. Ref.: https://goo.gl/UDoWY6
- Moore DS, McCabe GP, Craig B. Introducrion to the Practice of Statistics, 6th edition. W.H. Freeman & Company, St. Louis, MO, 2009.
- Tang Y, Ghosal S, Roy A. Nonparametric Bayesian estimation of positive false discovery rates. Biometrics. 2007; 63: 1126-1134. Ref.: https://goo.gl/fhT11d
- Nagan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, et al. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. 2007; 95: 652-662. Ref.: https://goo.gl/5Wr9zQ
- Yildiz OY, Aslan A, Alpagdin E. Multivariate statistical tests for comparing classification algorithms. In Learning and Intelligence Optimization. Springer. 2011.
- Reiner A, Yekutieli D, Benjamini Y. Identyfying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003; 19: 368-375. Ref.: https://goo.gl/zdjwUe
- Pawitan Y, Michiels S, Kosciely S, Gusnato A, Polner A. False discovery rate, sensitivity and sample size for microarray studies. Bioinformatics. 2005; 21: 3017-3024. Ref.: https://goo.gl/MDCtsB
- Choi H, Nesvizhskii AI. False discovery rates and related statistical concepts in mass spectrometry-based proteomics. J Proteome Res. 2008; 7: 47-50. Ref.: https://goo.gl/jEKHGT
- Earl-Slatter A. Cross Validation, In the Handbookmof Clinical Trials and Other Research. Radcliff Medical Press Ltd. 2002.
- Efron B, Tibshirani RJ. An introduction to the Bootstrap, Chapman and Hall. 1993.
- Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982; 143: 29-36. Ref.: https://goo.gl/rWnWhk
- Ringer M. What is principal component analysis? Nature Biotechnol. 2008; 26: 303-304. Ref.: https://goo.gl/F3nW3g
- Wegman E. Hyperdimensional data analysis using parallel coordinate. J Am Stat Assoc. 1990; 85: 644-675. Ref.: https://goo.gl/rZjzfv
- Gabriel KR, Odoroff CL. Biplots in biomedical research. Stat Med. 1990; 9: 469-485. Ref.: https://goo.gl/BCWcBJ
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protocol. 2009; 4: 44-57. Ref.: https://goo.gl/Ef9K2k
- Irizarry RA, Ladd-Acosta C, Wen B, Wu Z, Montano C, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserveds tissue-specific CpG island shores. Nat Genet. 2009; 41: 178-186. Ref.: https://goo.gl/5tpHH5
- Herman JG, Baylin SB. Gene silencing in association with promoter hypermethylation. N Eng J Med. 2003; 349: 2042-2054. Ref.: https://goo.gl/CwmWdN
- Hansen KD, Timp W, Corrada H, Sabunciyan S, Langmead B, et al. Increased methylation variation in epigenetic domains across cancer types. Nature Genet. 2011; 43: 768-775. Ref.: https://goo.gl/d4heLS
- Sarver AL, French AJ, Borralho PM, Thayanithy V, Oberg AL, et al. Human colon cancer profiles show differential microRNA expression depending on mismatch repair status and are characteristic of undifferentiated proliferative states. BMC Cancer. 2009; 9: 401. Ref.: https://goo.gl/r48pYJ
- Earle JS, Luthra R, Romans A, Abraham R, Ensor J, et al. Association of microRNA expression with microsatellite instability status in colorectal adenocarcinoma. J Mol Diag. 2010; 12: 433-440. Ref.: https://goo.gl/Mzn7aC
- Balaguer F, Moreira L, Lozano JJ, Link A, Ramirez G, et al. Colorectal cancers with microsatellite instability display unique miRNA profiles. Clin Cancer Res. 2011; 17: 6239-6249. Ref.: https://goo.gl/Xj5m2D
- Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, et al. The cartlidge specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006; 580: 4214-4217. Ref.: https://goo.gl/uCPD9h
- Costa Y, Speed RM, Gautier P, Semple CA, Maratou K, et al. Mouse MAELSTROM: the link between miotic silencing of unsynapsed chromatin and microRNA pathways? Hum Mol Genet. 2006; 15: 2324-2334. Ref.: https://goo.gl/g5ApSb
- Rajewsky N. microRNA target predictions in animals. Nat Genet. 2006; 38: S8-S13. Ref.: https://goo.gl/1Y3BRr
- Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, et al. Specific activation of microRNA-127 with downregulation of the protooncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006; 9: 435-443. Ref.: https://goo.gl/4CvfGe
- Lujambio A, Calin GA, Villanueva A, Ropero S, Sánchez-Céspedes M, et al. A microRNA DNA methylation signature for human cancer metastasis. Proc Natl Acad Sci USA. 2008; 105: 13556-13561. Ref.: https://goo.gl/czoFWV
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, et al. Augmentation of tumor angiogenesis by a myc-activated microRNA cluster. Nature Genet. 2006; 38: 1060-1065. Ref.: https://goo.gl/P2kXXb
- Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, et al. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005; 123: 819-831. Ref.: https://goo.gl/c2ogai
- Koss LG, Melamed MR, Eds. Koss’ Diagnostic Cytology and Histopathologic Bases, 5th edition, Lippincott, Williams & Wilkins, 2005.
- Winter MJ, Nagtegaal ID, van Krieken JH, Litvinov SV. The epithelial cell adhesion molecule (Ep-CAM) as a morphoregulatory molecule is a tool in surgical pathology. Am J Pathol. 2003; 163: 2139-2148. Ref.: https://goo.gl/9qiDgf
- Petrelli NJ, Letourneau R, Weber T, Nava ME, Rodriguez-Bigas M. Accuracy of biopsy and cytology for the preoperative diagnosis of colorectal adenocarcinoma. J Surg Oncol. 1999; 71: 46-49. Ref.: https://goo.gl/KsPfWB
- Matsushita HM, Matsumura Y, Moriya Y, Akasu T, Fujita S, et al. A new method for isolating colonocytes from naturally evacuated feces and its clinical application to colorectal cancer diagnosis. Gastroenterology. 2005; 129: 1918 - 1927. Ref.: https://goo.gl/mZAy84
- Simpson RJ, Lim JE, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009; 6: 267-283. Ref.: https://goo.gl/RR1Xtf
- Baker M. Digital PCR hits its stride. Nature Methods. 2012; 9: 541-544. Ref.: https://goo.gl/FQfNH5
- McShane LM, Altman DG, Sauerbrei W, Sheila E. Taube, Massimo Gion, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005; 97: 1180-1184. Ref.: https://goo.gl/nGLTAy
Figures:

Figure 1

Figure 2

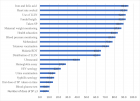
Figure 3

Figure 4
Similar Articles
-
Surface Plasmon Resonance technology to assess biological interactionsSilvia Bartollino*,Alessandro Medoro,Donatella Mignogna,,Erika di Zazzo,Bruno Moncharmont. Surface Plasmon Resonance technology to assess biological interactions . . 2017 doi: 10.29328/journal.hjbm.1001005; 1: 039-044
-
Use of MicroRNAs to Screen for Colon CancerFarid E Ahmed*,Nancy C Ahmed,Mostafa Gouda,Chris Bonnerup. Use of MicroRNAs to Screen for Colon Cancer . . 2017 doi: 10.29328/journal.hjbm.1001006; 1: 045-074
Recently Viewed
-
Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle GlaucomaKonstantinos Kyratzoglou*,Katie Morton. Cystoid Macular Oedema Secondary to Bimatoprost in a Patient with Primary Open Angle Glaucoma. Int J Clin Exp Ophthalmol. 2025: doi: 10.29328/journal.ijceo.1001059; 9: 001-003
-
Metastatic Brain Melanoma: A Rare Case with Review of LiteratureNeha Singh,Gaurav Raj,Akshay Kumar,Deepak Kumar Singh,Shivansh Dixit,Kaustubh Gupta*. Metastatic Brain Melanoma: A Rare Case with Review of Literature. J Radiol Oncol. 2025: doi: ; 9: 050-053
-
Depression as a civilization-deformed adaptation and defence mechanismBohdan Wasilewski*,Olha Yourtsenyuk,Eugene Egan. Depression as a civilization-deformed adaptation and defence mechanism. Insights Depress Anxiety. 2020: doi: 10.29328/journal.ida.1001013; 4: 008-011
-
Drinking-water Quality Assessment in Selective Schools from the Mount LebanonWalaa Diab, Mona Farhat, Marwa Rammal, Chaden Moussa Haidar*, Ali Yaacoub, Alaa Hamzeh. Drinking-water Quality Assessment in Selective Schools from the Mount Lebanon. Ann Civil Environ Eng. 2024: doi: 10.29328/journal.acee.1001061; 8: 018-024
-
Rapid Microbial Growth in Reusable Drinking Water BottlesQishan Liu*,Hongjun Liu. Rapid Microbial Growth in Reusable Drinking Water Bottles. Ann Civil Environ Eng. 2017: doi: 10.29328/journal.acee.1001007; 1: 055-062
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."