Abstract
Review Article
Synthetic Animal: Trends in Animal Breeding and Genetics
Abolfazl Bahrami* and Ali Najafi
Published: 11 January, 2019 | Volume 3 - Issue 1 | Pages: 007-025
Synthetic biology is an interdisciplinary branch of biology and engineering. The subject combines various disciplines from within these domains, such as biotechnology, evolutionary biology, molecular biology, systems biology, biophysics, computer engineering, and genetic engineering. Synthetic biology aims to understand whole biological systems working as a unit, rather than investigating their individual components and design new genome. Significant advances have been made using systems biology and synthetic biology approaches, especially in the field of bacterial and eukaryotic cells. Similarly, progress is being made with ‘synthetic approaches’ in genetics and animal sciences, providing exciting opportunities to modulate, genome design and finally synthesis animal for favorite traits.
Read Full Article HTML DOI: 10.29328/journal.ibm.1001015 Cite this Article Read Full Article PDF
Keywords:
Synthetic biology; Systems biology; Synthetic approaches; Genetic engineering
References
- Charles D. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. Nature. 1859; 5: 502. Ref.: https://goo.gl/Vd9Zda
- Wright S. Statistical genetics and evolution. Bull Amer Math Soc 1942; 48: 223–246. Ref.: https://goo.gl/kFP84Y
- Fisher RA. The Correlation between Relatives on the Supposition of Mendelian Inheritance. Philosophical Transactions of the Royal Society of Edinburgh. 1918; 52: 399–433. Ref.: https://goo.gl/1FTvrH
- Haldane JB. Linkage in poultry. Science. 1921; 54: 663. Ref.: https://goo.gl/ayCNS9
- Morgan TH. Sex-limited inheritance in Drosophila. Science. 1910; 32:120–122. Ref.: https://goo.gl/cpCDXv
- Lush JL. 1896 - 1982 Biographical Memoirs of the AAAS. Ref.: https://goo.gl/C133gz
- Van Vleck LD. Charles Roy Henderson, 1911-1989: A brief biography. J Anim Sci. 1998; 76: 2959–2961. Ref.: https://goo.gl/Gjhraa
- Bahrami A, Miraei-Ashtiani SR, Mehrabani-Yeganeh H. Associations of growth hormone secretagogue receptor (GHSR) genes polymorphisms and protein structure changes with carcass traits in sheep. Gene. 2012; 505: 379–383. Ref.: https://goo.gl/GZy8PK
- Bahrami A, Behzadi SH, Miraei-Ashtiani SR, Roh SG, Katoh K. Genetic polymorphisms and protein structures in growth hormone, growth hormone receptor, ghrelin, insulin-like growth factor 1 and leptin in Mehraban sheep. Gene. 2013; 527: 397–404. Ref.: https://goo.gl/sZu7RM
- Meuwissen TH, Goddard ME. Accurate prediction of genetic values for complex traits by whole-genome resequencing. Genetics. 2010; 185: 623–631. Ref.: https://goo.gl/2jynnU
- Meuwissen TH, Hayes BJ, Goddard ME. Prediction of total genetic value using genome-wide dense marker maps. Genetics. 2001; 157: 1819–1829. Ref.: https://goo.gl/CdLnVe
- Cole JB, VanRaden PM, O'Connell JR, Van Tassell CP, et al. Distribution and location of genetic effects for dairy traits. J Dairy Sci. 2009; 92: 2931–2946. Ref.: https://goo.gl/Jucx4Q
- Daetwyler HD, Kemper KE, van der Werf JH, Hayes BJ. Components of the accuracy of genomic prediction in a multi-breed sheep population. J Anim Sci 2012; 90: 3375–3384. Ref.: https://goo.gl/mhxhnB
- Pryce JE, Daetwyler HD. Designing dairy cattle breeding schemes under genomic selection: a review of international research. Anim Prod Sci. 2011; 52: 107–114. Ref.: https://goo.gl/tfrgDu
- Schaeffer LR. Strategy for applying genome-wide selection in dairy cattle. J Anim Breed Genet. 2006; 123: 218–223. Ref.: https://goo.gl/iCbuqF
- Erbe M, Hayes BJ, Matukumalli LK, Goswami S, Bowman PJ, et al., Improving accuracy of genomic predictions within and between dairy cattle breeds with imputed high-density single nucleotide polymorphism panels. J Dairy Sci. 2012; 95: 4114–4129. Ref.: https://goo.gl/XfWQiV
- Daetwyler HD, Villanueva B, Woolliams JA. Accuracy of predicting the genetic risk of disease using a genome-wide approach. PLoS ONE. 2008; 3: e3395. Ref.: https://goo.gl/JPHkUa
- Goddard M. Genomic selection: prediction of accuracy and maximisation of long term response. Genetica. 2008; 136: 245-257. Ref.: https://goo.gl/SpuwtD
- Habier D, Tetens J, Seefried FR, Lichtner P, Thaller G. The impact of genetic relationship information on genomic breeding values in German Holstein cattle. Genet Sel Evol. 2010; 42: 5. Ref.: https://goo.gl/QUYg71
- Hayes BJ, Macleod I, Daetwyler MD, Goddard ME. Towards genomic prediction from genome sequence data and the 1000 bull genomes project, Proceedings 4th International Conference on Quantiative Genetics, Edinburgh. 2012; O–54. Ref.: https://goo.gl/rg4DZJ
- Sanford JC, Klein TM, Wolf ED, Allen N. Delivery of substances into cells and tissues using a particle bombardment process. Journal of Particulate Science and Technology. 1987; 5: 27–37. Ref.: https://goo.gl/FXPGpK
- Klein RM, Wolf ED, Wu R, Sanford JC. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature. 1987; 327: 70–73. Ref.: https://goo.gl/dApfMA
- Park F. Lentiviral vectors: are they the future of animal transgenesis? Physiol. Genomics. 2007; 31: 159–173. Ref.: https://goo.gl/2aqAeY
- Lee LY, Gelvin SB. T-DNA binary vectors and systems. Plant Physiol. 2008; 146: 325–332. Ref.: https://goo.gl/qamdgR
- Jackson DA, Symons RH, Berg P. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia coli. PNAS. 1972; 69: 2904–2909. Ref.: https://goo.gl/YctKZY
- Brophy B, Smolenski G, Wheeler T, Wells D, L'Huillier P, et al. Cloned transgenic cattle produce milk with higher levels of β-casein and κ-casein. Nat Biotechnol. 2003; 21; 157–162. Ref.: https://goo.gl/J24QzX
- Clark J. The Mammary Gland as a Bioreactor: Expression, Processing, and Production of Recombinant Proteins. Journal of Mammary Gland Biology and Neoplasia. 1998; 3: 337–350. Ref.: https://goo.gl/EJfyn2
- Gordon K, Lee E, Vitale JA, Smith AE, Westphal H, et al. Production of human tissue plasmnogen activator in transgenic mouse milk. Biotechnology. 1987; 5: 1183-1187. Ref.: https://goo.gl/iqhpp6
- Anastasia B. Risk Assessment and Mitigation of AquAdvantage Salmon. 2010; ISB News Report. Ref.: https://goo.gl/Jjxcyw
- Thomas MA, Roemer GW, Donlan CJ, Dickson BG, Matocq M, et al. Ecology: Gene tweaking for conservation. Nature. 2013; 501: 485–486. Ref.: https://goo.gl/GtDny1
- Jaenisch R, Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad. 1974; 71: 1250–1254. Ref.: https://goo.gl/j3DBBS
- Sathasivam K, Hobbs C, Mangiarini L, Mahal A, Turmaine M, et al. Transgenic models of Huntington's disease. Philos Trans R Soc Lond B Biol Sci. 1999; 354: 963–969. Ref.: https://goo.gl/7LR7Jo
- Spencer LT, Humphries JE, Brantly ML; Transgenic Human Alpha 1-Antitrypsin Study Group. Antibody Response to Aerosolized Transgenic Human Alpha1-Antitrypsin. N Engl J Med. 2005; 352: 2030. Ref.: https://goo.gl/zgqVM4
- Schatten G, Mitalipov S. Developmental biology: Transgenic primate offspring. Nature. 2009; 459: 515–516. Ref.: https://goo.gl/nfzPnV
- Richard G. Genetically modified cows produce 'human' milk. 2011; Ref.: https://goo.gl/QaBjjC
- Wagner JS, McCracken J, Wells DN, Laible G, Targeted microRNA expression in dairy cattle directs production of -lactoglobulin-free, high-casein milk. Proceedings of the National Academy of Sciences. 2012; 109: 16811–16816. Ref.: https://goo.gl/oaZ6hT
- Margawati ET. Transgenic Animals: Their Benefits To Human Welfare. Actionbioscience. Retrieved June 29, 2014; Ref.: https://goo.gl/yvMECq
- Capecchi MR. Gene targeting in mice: functional analysis of the mammalian genome for the twenty-first century. Nat Rev Genet. 2005; 6: 507–512. Ref.: https://goo.gl/xeXiqP
- Cong L, Ran FA, Cox D, Lin S, Barretto R, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013; 339: 819–823. Ref.: https://goo.gl/QkraAU
- DiCarlo JE, Norville JE, Mali P, Rios X, Aach J, et al. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 2013; 41: 4336–4343. Ref.: https://goo.gl/rT4FKq
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, et al. RNA-guided human genome engineering via Cas9. Science. 2013; 339: 823–826. Ref.: https://goo.gl/keJNi3
- Friedland AE, Tzur YB, Esvelt KM, Colaiácovo MP, Church GM, et al. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat Methods. 2013; 10: 741–743. Ref.: https://goo.gl/QV1akB
- Xue H, Wu J, Li S, Rao MS, Liu Y. Genetic Modification in Human Pluripotent Stem Cells by Homologous Recombination and CRISPR/Cas9 System. Methods Mol Biol. 2016; 1307:173-190. Ref.: https://goo.gl/TtwWqh
- Esvelt KM, Wang HH. Genome-scale engineering for systems and synthetic biology. Mol Syst Biol. 2013; 9: 641. Ref.: https://goo.gl/yFaS15
- Ling MM, Robinson BH. Approaches to DNA mutagenesis: an overview, Analytical Biochemistry. 1997; 254: 157–178. Ref.: https://goo.gl/ayHjC4
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989; 244: 1288-1292. Ref.: https://goo.gl/vZhv6s
- de Souza N. Primer: genome editing with engineered nucleases. Nat Meth. 2011; 9: 27-27. Ref.: https://goo.gl/zT5kkz
- Chevalier BS, Kortemme T, Chadsey MS, Baker D, Monnat RJ, et al. Design, Activity, and Structure of a Highly Specific Artificial Endonuclease. Molecular Cell. 2002; 10: 895-905. Ref.: https://goo.gl/GuDWgo
- Smith J, Grizot S, Arnould S, Duclert A, Epinat JC, et al. A combinatorial approach to create artificial homing endonucleases cleaving chosen sequences. Nucleic Acids Research. 2006; 34: e149. Ref.: https://goo.gl/KMHBAH
- Baker M. Gene-editing nucleases. Nat Meth. 2012; 9: 23-26. Ref.: https://goo.gl/qoViMw
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010; 11: 636-646. Ref.: https://goo.gl/gYL6WE
- Boissel S, Jarjour J, Astrakhan A, Adey A, Gouble A, et al. megaTALs: a rare-cleaving nuclease architecture for therapeutic genome engineering. Nucleic Acids Research. 2014; 42: 2591–2601. Ref.: https://goo.gl/hEuSdm
- Bahrami A, Miraie-Ashtiani SR, Sadeghi M, Najafi A. miRNA-mRNA network involved in folliculogenesis interactome: systems biology approach. Reproduction. 2017; 154: 51-65. Ref.: https://goo.gl/cVfrhx
- Bahrami A, Miraie-Ashtiani SR, Sadeghi M, Najafi A, Ranjbar R. Dynamic modeling of folliculogenesis signaling pathways in the presence of miRNAs expression. J Ovarian Res. 2017; 10: 76. Ref.: https://goo.gl/LrNcDQ
- Alberghina L, Westerhoff HV. Systems Biology: Definitions and Perspectives. Topics in Current Genetics. 2005; 13: 13–30. Ref.: https://goo.gl/zYUL73
- Kholodenko BN, Sauro HM, eds. Systems Biology: Definitions and Perspectives. Topics in Current Genetics. 2005; 13: 357–451.
- Chiara R, Gerolamo L. Statistical Tools for Gene Expression Analysis and Systems Biology and Related Web Resources. In Stephen Krawetz, Bioinformatics for Systems Biology. 2009; Humana Press:. 181–205. Ref.: https://goo.gl/Jyuatn
- von Bertalanffy L. General System theory: Foundations, Development, Applications. George Braziller. 1976; 295. Ref.: https://goo.gl/d3kjVH
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952; 117: 500–544. Ref.: https://goo.gl/KTq2ER
- Noble D. Cardiac action and pacemaker potentials based on the Hodgkin-Huxley equations. Nature.1960; 188: 495–497. Ref.: https://goo.gl/4w7HtK
- Rosen R. A Means toward a New Holism. Science. 1968; 161: 34–35. Ref.: https://goo.gl/USthvk
- Hunter P. Back down to Earth: Even if it has not yet lived up to its promises, systems biology has now matured and is about to deliver its first results. EMBO Reports. 2012; 13: 408–411. Ref.: https://goo.gl/7eoD7E
- Zeng BJ. On the concept of system biological engineering. Communication on Transgenic Animals. 1994a; 6.
- Zeng BJ. Transgenic animal expression system – transgenic egg plan (goldegg plan). Communication on Transgenic Animals. 1994b; 1:11
- Zeng BJ. From positive to synthetic science. Communication on Transgenic Animals. 1995; 11.
- Tomita M, Hashimoto K, Takahashi K, Shimizu TS, Matsuzaki Y, et al. E-CELL: Software Environment for Whole Cell Simulation,. Genome Inform Ser Workshop Genome Inform. 199; P 8: 147–155. Ref.: https://goo.gl/vRcbX5
- Karr JR, Sanghvi JC, Macklin DN, Gutschow MV, Jacobs JM, et al. A Whole-Cell Computational Model Predicts Phenotype from Genotype. Cell. 2012; 150: 389–401. Ref.: https://goo.gl/H9dwgF
- Tavassoly I. Dynamics of Cell Fate Decision Mediated by the Interplay of Autophagy and Apoptosis in Cancer Cells. Springer International Publishing. ISBN. 2015; 978-3-319-14961-5. Ref.: https://goo.gl/T5GmRj
- Nakano T. Molecular Communication. Cambridge. ISBN. 2013, 978-1-107-02308-6. Ref.: https://goo.gl/EsqF63
- Elowitz MB, Leibler S. A synthetic oscillatory network of transcriptional regulators. Nature. 2000; 403: 335–338. Ref.: https://goo.gl/Nw8FLz
- Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000; 403: 339–342. Ref.: https://goo.gl/Bmkvyg
- Channon K, Bromley EH, Woolfson DN. Synthetic Biology through Biomolecular Design and Engineering. Curr Opin Struct Biol. 2008; 18: 491–498. Ref.: https://goo.gl/MpkfdD
- Stone M. Life Redesigned to Suit the Engineering Crowd. Microbe. 2006; 1: 566–570. Ref.: https://goo.gl/HkEBp2
- Zhang R, Lin Y. DEG 5.0, a database of essential genes in both prokaryotes and eukaryotes. Nucleic Acids Res. 2009; 37: D455–D458. Ref.: https://goo.gl/dmRreS
- Juhas M, Eberl L, Glass JI. Essence of life: Essential genes of minimal genomes. Trends Cell Biol. 2011; 21: 562–568. Ref.: https://goo.gl/zt5r8q
- Hutchison CA, Peterson SN, Gill SR, Cline RT, White O, et al., Global transposon mutagenesis and a minimal Myco- plasma genome. Science. 1999; 286: 2165–2169. Ref.: https://goo.gl/tU1oag
- Goodman AL, Wu M, Gordon JI. Identifying microbial fitness determinants by insertion sequencing using genome-wide transposon mutant libraries. Nat Protoc. 2011; 6: 1969 –1980. Ref.: https://goo.gl/oh2yj1
- van Opijnen T, Bodi KL, Camilli A. Tn-seq: High-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009; 6: 767–772. Ref.: https://goo.gl/p1bJZf
- Christen B, Abeliuk E, Collier JM, Kalogeraki VS, Passarelli B, et al. The essential genome of a bacterium. Mol Syst Biol. 2011; 7: 528. Ref.: https://goo.gl/PRdo5h
- Luo H, Lin Y, Gao F, Zhang CT, Zhang R. DEG 10, an update of the database of essential genes that includes both protein-coding genes and noncoding genomic elements. Nucleic Acids Res. 2014; 42: D574–D580. Ref.: https://goo.gl/15WnQ7
- Wetmore KM, Price MN, Waters RJ, Lamson JS, He J, et al. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. MBio. 2015; 6: e00306-15. Ref.: https://goo.gl/cDDxcR
- Zhang R, Patena W, Armbruster U, Gang SS, Blum SR, et al. High-throughput genotyping of green algal mutants reveals random distribution of mutagenic insertion sites and endonucleolytic cleavage of transforming DNA. Plant Cell. 2014; 26: 1398–1409. Ref.: https://goo.gl/NwYcc2
- Angermayr SA, Gorchs Rovira A, Hellingwerf KJ. Metabolic engineering of cyanobacteria for the synthesis of commodity products. Trends Biotechnol. 2015; 33: 352–361. Ref.: https://goo.gl/QsVikZ
- Basulto D. Everything you need to know about why CRISPR is such a hot technology. Washington Post. 2015 Retrieved 5 December.
- Rollié S, Mangold M, Sundmacher K. Designing biological systems: Systems Engineering meets Synthetic Biology. Chemical Engineering Science. 2012; 69: 1–29. Ref.: https://goo.gl/AiVUFu
- Kaznessis YN. Models for synthetic biology. BMC Systems Biology. 2007; 1: 47. Ref.: https://goo.gl/ze2Sdr
- Masoudi-Nejad A, Bidkhori G2, Hosseini Ashtiani S2, Najafi A2, Bozorgmehr JH, et al. Cancer systems biology and modeling: microscopic scale and multiscale approaches. Semin. Cancer Biol. 2015; 30: 60–69. Ref.: https://goo.gl/LZwrco
- Najafi A, Bidkhori G, Bozorgmehr JH, Koch I, Masoudi-Nejad A. Genome scale modeling in systems biology: algorithms and resources. Curr. Genomics. 2014; 15: 130–159. Ref.: https://goo.gl/KXqKHi
- Kosuri S, Church GM. Large-scale de novo DNA synthesis: technologies and applications. Nature Methods. 2014; 11: 499–507. Ref.: https://goo.gl/SHRaZY
- Blight KJ, Kolykhalov AA, Rice CM. Efficient initiation of HCV RNA replication in cell culture. Science. 2000; 290: 1972–1974. Ref.: https://goo.gl/j344wQ
- Smith HO, Hutchison CA 3rd, Pfannkoch C, Venter JC. Generating a synthetic genome by whole genome assembly: {phi} X174 bacteriophage from synthetic oligonucleotides. Proc Natl Acad Sci USA. 2003; 100: 15440–15445. Ref.: https://goo.gl/ggr443
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science.2008; 319: 1215–1220. Ref.: https://goo.gl/h3bj48
- Kramer BP, Fischer C, Fussenegger M. Biologic gates enable logical transcription control in mammalian cells. Biotechnol. Bioeng. 2004; 87: 478–484. Ref.: https://goo.gl/6NcbQL
- Nissim L, Bar-Ziv RH. A tunable dual-promoter integrator for targeting of cancer cells. Mol Syst Biol. 2010; 6: 444. Ref.: https://goo.gl/9KgZ27
- Lohmueller JJ, Armel TZ, Silver PA. A tunable zinc finger-based framework for Boolean logic computation in mammalian cells. Nucleic Acids Res. 2012; 40: 5180–5187. Ref.: https://goo.gl/aaS2eL
- Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013, 152: 1173–1183. Ref.: https://goo.gl/xE5Pzc
- Maeder ML, Linder SJ, Cascio VM, Fu Y, Ho QH, et al. CRISPR RNA-guided activation of endogenous human genes. Nat Methods. 2013; 10: 977–979. Ref.: https://goo.gl/zHZdkq
- Kiani S, Beal J, Ebrahimkhani MR, Huh J, Hall RN, et al. CRISPR transcriptional repression devices and layered circuits in mammalian cells. Nat Methods. 2014; 11: 723–726. Ref.: https://goo.gl/EE86T4
- Nissim L, Perli SD, Fridkin A, Perez-Pinera P, Lu TK. Multiplexed and programmable regulation of gene networks with an integrated RNA and CRISPR/ CAS toolkit in human cells. Mol Cell. 2014; 54: 698–710. Ref.: https://goo.gl/LVgoyy
- Fussenegger M, Morris RP, Fux C, Rimann M, von Stockar B, et al. Streptogramin-based gene regulation systems for mammalian cells. Nat Biotechnol. 2000; 18: 1203–1208. Ref.: https://goo.gl/kGbh64
- Gillette MU, Sejnowski TJ. Physiology: biological clocks coordinately keep life on time. Science. 2005; 309: 1196–1198. Ref.: https://goo.gl/Bs48Gc
- Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004; 430: 467–471. Ref.: https://goo.gl/YF6FNt
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappa B activation. Science. 2005; 309: 1854–1857. Ref.: https://goo.gl/wCS2Qk
- Lahav G. The strength of indecisiveness: oscillatory behavior for better cell fate determination. Sci STKE. 2004; 55. Ref.: https://goo.gl/ufX2Y8
- Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M. A tunable synthetic mammalian oscillator. Nature. 2009; 457: 309–312. Ref.: https://goo.gl/GseoBW
- Tigges M, Dénervaud N, Greber D, Stelling J, Fussenegger M. A synthetic low-frequency mammalian oscillator. Nucleic Acids Res. 2010; 38: 2702–2711. Ref.: https://goo.gl/SZy5fZ
- Stricker J, Cookson S, Bennett MR, Mather WH, Tsimring LS, et al. A fast, robust and tunable synthetic gene oscillator. Nature. 2008; 456: 516–519. Ref.: https://goo.gl/LPSYtK
- Ausländer S, Ausländer D, Müller M, Wieland M, Fussenegger M. Programmable single-cell mammalian biocomputers. Nature. 2012; 487: 123–127. Ref.: https://goo.gl/uU1EVR
- Montague MG, Lartigue C, Vashee S. Synthetic genomics: potential and limitations. Current Opinion in Biotechnology. 2012; 23: 659-665. Ref.: https://goo.gl/qa5AYx
- Deamer A. giant step towards artificial life? Trends Biotechnol. 2005; 23: 336–338. Ref.: https://goo.gl/4RkuQ6
- Malyshev DA, Dhami K, Lavergne T, Chen T, Dai N, et al. A semi-synthetic organism with an expanded genetic alphabet. Nature. 2014; 509: 385–388. Ref.: https://goo.gl/98PDPH
- Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, et al. Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science. 2010; 329: 52–56. Ref.: https://goo.gl/RTEw3p
- Rogers-Hayden T, Pidgeon N. Reflecting upon the UK’s Citizens’ Jury on Nanotechnologies: Nano Jury UK. Nanotechnology Law & Business. 2006; 167-178. Ref.: https://goo.gl/6dmCpM
- Wynne B. Creating Public Alienation: Expert Cultures of Risk and Ethics on GMOs. Sci Cult (Lond). 2001; 10: 445-481. Ref.: https://goo.gl/JWk4vh
- Gregory R, Fischhoff B, McDaniels T. Acceptable Input: Using Decision Analysis to Guide Public Policy Deliberations. Decision Analysis. 2005; 2: 4-16. Ref.: https://goo.gl/5hhYx2
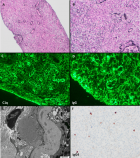
Figures:
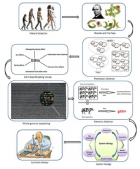
Figure 1
Similar Articles
-
Synthetic Animal: Trends in Animal Breeding and GeneticsAbolfazl Bahrami*,Ali Najafi. Synthetic Animal: Trends in Animal Breeding and Genetics. . 2019 doi: 10.29328/journal.ibm.1001015; 3: 007-025
Recently Viewed
-
A Gateway to Metal Resistance: Bacterial Response to Heavy Metal Toxicity in the Biological EnvironmentLoai Aljerf*,Nuha AlMasri. A Gateway to Metal Resistance: Bacterial Response to Heavy Metal Toxicity in the Biological Environment. Ann Adv Chem. 2018: doi: 10.29328/journal.aac.1001012; 2: 032-044
-
Obesity in Patients with Chronic Obstructive Pulmonary Disease as a Separate Clinical PhenotypeDaria A Prokonich*, Tatiana V Saprina, Ekaterina B Bukreeva. Obesity in Patients with Chronic Obstructive Pulmonary Disease as a Separate Clinical Phenotype. J Pulmonol Respir Res. 2024: doi: 10.29328/journal.jprr.1001060; 8: 053-055
-
Current Practices for Severe Alpha-1 Antitrypsin Deficiency Associated COPD and EmphysemaMJ Nicholson*, M Seigo. Current Practices for Severe Alpha-1 Antitrypsin Deficiency Associated COPD and Emphysema. J Pulmonol Respir Res. 2024: doi: 10.29328/journal.jprr.1001058; 8: 044-047
-
Navigating Neurodegenerative Disorders: A Comprehensive Review of Current and Emerging Therapies for Neurodegenerative DisordersShashikant Kharat*, Sanjana Mali*, Gayatri Korade, Rakhi Gaykar. Navigating Neurodegenerative Disorders: A Comprehensive Review of Current and Emerging Therapies for Neurodegenerative Disorders. J Neurosci Neurol Disord. 2024: doi: 10.29328/journal.jnnd.1001095; 8: 033-046
-
Metastatic Brain Melanoma: A Rare Case with Review of LiteratureNeha Singh,Gaurav Raj,Akshay Kumar,Deepak Kumar Singh,Shivansh Dixit,Kaustubh Gupta*. Metastatic Brain Melanoma: A Rare Case with Review of Literature. J Radiol Oncol. 2025: doi: 10.29328/journal.jro.1001080; 9: 050-053
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."