More Information
Submitted: 20 April 2020 | Approved: 29 April 2020 | Published: 30 April 2020
How to cite this article: Shkal KEM, Azab AE, Attia AM, El-Banna SG, Yahya RAM. Zinc oxide nanoparticles attenuate the oxidative damage and disturbance in antioxidant defense system induced by cyclophosphamide in male albino rats. Insights Biol Med. 2020; 4: 001-008.
DOI: 10.29328/journal.ibm.1001016
ORCiD: orcid.org/0000-0002-2974-0219
Copyright License: © 2020 Shkal KEM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Cyclophosphamide; Zinc oxide nanoparticles; Attenuation; Ameliorative effect; Oxidative damage; Antioxidant defense system
Zinc oxide nanoparticles attenuate the oxidative damage and disturbance in antioxidant defense system induced by cyclophosphamide in male albino rats
Karema El M Shkal1, Azab Elsayed Azab2*, Ahmed M Attia3, Sabah G El-Banna3 and Rabia AM Yahya1
1Pharmacology Department, Faculty of Medicine, Sabratha University, Libya
2Physiology Department, Faculty of Medicine, Sabratha University, Libya
3Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Egypt
*Address for Correspondence: Azab Elsayed Azab, Physiology Department, Faculty of Medicine, Sabratha University, Libya, Tel: 00218925674752; Email: [email protected]
Background: Cyclophosphamide is used for the treatment of malignant and non-malignant diseases, but, it induces oxidative damage and disturbance in the antioxidant defense system. Zinc oxide nanoparticles (ZnO NPs) are used in biomedical applications and consumer products. ZnO-NPs are protected cell membranes against oxidative damage, decrease free radicals and malondialdehyde (MDA) levels, and increase the antioxidant enzyme levels.
Objectives: The present aimed to evaluate the ameliorative effect of Zn-O nano-particles on oxidative damage and disturbance in the antioxidant defense system induced by cyclophosphamide in male albino rats.
Materials and Methods: 24 adult male albino rats were randomly divided into 4 groups (6 rats of each). Group I (Control group): Received 0.2 ml saline /day i.p. injection for 14 days (day by day), group II, (nZnO group): Received nZnO (5 mg/kg/day) b.w., intraperitoneally for 14 days, Group III (CP group): Received CP (20 mg/kg/day) b.w, day by day for 14 days by intraperitoneal injection, Group IV (CP + ZnO NPs group): Received nZnO group: Received nZnO (5 mg/kg/day) b.w., intraperitoneally for 14 days, plus CP (20 mg/kg/day) b.w., day by day for 14 days by intraperitoneal injection. After 24-hr from the last treatment, all animals were anesthetized using light ether. Blood, lungs, and liver samples were taken and prepared for biochemical measurements.
Results: Individual treatment of zinc oxide nanoparticles and CP induced liver cytochrome b5, cytochrome C reductase, and glutathione S-transferase (GST) compared to the control group, while CP increased P450. The combination of nZnO and CP prevents the elevation of cytochrome b5, P450, cytochrome C reductase, and GST compared with the CP treated group. Zinc oxide nanoparticles and CP increased liver thiobarbituric acid reactive substances (TBARS). The combination of nZnO and CP prevents the changes in TBARS concentrations compared with the CP. Injection of CP to rats reduced the activities of serum glutathione reductase (GR) and catalase (CAT) as compared with the control group. However, combination treatment of rats with nZnO and CP increased the activities of these enzymes compared with those treated with CP alone. Zinc oxide nanoparticles and CP increased serum and lung TBARS, while decreased glutathione (GSH) concentration compared to the control group, with more pronounced changes by CP. The combination of nZnO and CP prevents the changes in TBARS and GSH concentrations compared with the CP.
Conclusion: It can be concluded that CP induced oxidative stress and disturbance in the antioxidant defense system. Treatment of rats with zinc oxide nano-particles and CP together attenuated the oxidative damage and disturbance in the antioxidant defense system induced by CP. So, Patients treated with CP advised to take nZnO to prevent the side effects of chemotherapy. Further studies are necessary to evaluate the amelioration effect nZnO and other nano-particles against oxidative stress induced by CP in different doses and experimental models.
Cyclophosphamide is used for the treatment of multiple myeloma, leukemia, lymphoma, vasculitis, and rheumatoid arthritis has been well-established [1]. Clinical use of CP is limited because of its toxicity associated with increased inflammations and oxidative stress [2]. CP is metabolized by the Cytochrome P-450 pathway. The toxicity of CP is required for bioactivation by hepatic microsomal cytochrome P-450 mixed functional oxidase yields, 4-hydroxycyclophosphamide which is existed in equilibrium with aldophosphamide, and is degraded by β-elimination to form an equimolar amount of byproduct acrolein and phosphoramide mustard [3,4]. Oxidative stress has linked to the cause of many diseases (chronic kidney disease, coronary artery disease, cerebrovascular disease, amyotrophic lateral sclerosis, heart failure, and neurodegenerative diseases [5-7]. It arises from an imbalance between reactive nitrogen species, insufficient antioxidant defense, and excessive generation of reactive oxygen species (ROS). It can lead to the progression of atherosclerosis by oxidative damage to cellular components. Reactive nitrogen species and excessive generation of reactive oxygen species are unstable and participate in the degradation of the cell membrane and deoxyribonucleic acid.
Schacter, et al. [8] found that the elevation of reactive oxygen species in sickle cells reduced the activity of superoxide dismutase (SOD). Also, Scott, et al. [9] concluded that H2O2 mediates cellular damage through .O2- generating systems and lead to a reduction in superoxide dismutase activity. Oxidative stress and inflammation are lead to tissue damage [10]. So, there is a need to reduce oxidative stress for the treatment of diseases. ZnO is widely used metal oxides in several numerous products like coatings, paints, and cosmetics products like sunscreens thanks to their antioxidant, anti-inflammatory, antimicrobial, and UV ray protecting properties [11-13]. ZnONPs are used in biomedical applications and consumer products. Human exposure to ZnONPs is a highly frequent and they have become an issue of concern to public health.
The protective effect of ZnONPs on oxidative stress-induced diabetes in male rats was studied by Hussein, et al. [14] who found that a significant increase in the serum concentrations of MDA and nitric oxide in the diabetic rats, and MDA and nitric oxide were significantly reduced post-treatment with ZnONPs. Also, Khalaf, et al. [13] recorded that ZnONPs cream was protected rats against oxidative damage and skin allergic dermatitis induced by lead oxide which may be due to the antioxidant properties and anti-inflammatory of Zinc oxide nanoparticles [13,15], and Zinc concentration was increased from the dissociation of Zinc oxide nanoparticles. Zinc is the core constituent of antioxidant enzymes (as superoxide dismutase) and it is a protector for sulfhydryl groups and impair lipid peroxidation by displacing transition metals (as copper and iron) from catalytic sites [13,16]. El-Maddawy and abd El Naby, [17] reported that rats treated male with doxorubicin showed significant decreases in catalase and glutathione activities and an increase in the testicular malondialdehyde concentration, which indicator of the increase in oxidative damage and lipid peroxidation caused by reactive oxygen species. Co-administration of zinc oxide nanoparticles (3 mg/kg b.w) plus doxorubicin induced a significant increased catalase and glutathione activities and significantly decrease in the testicular malondialdehyde concentration as compared with the doxorubicin-treated group. Zinc oxide nanoparticles possess antioxidant activity concerning mechanisms of both free radical scavenging and reducing activities [18]. Also, zinc oxide nanoparticles are protected cell membranes against oxidative damage, decrease free radicals and malondialdehyde (MDA) levels, and increase the antioxidant enzyme levels [13,17,19].
Objective
The present aimed to evaluate the ameliorative effect of Zn-O nano-particles on oxidative damage and disturbance in the antioxidant defense system induced by cyclophosphamide in male albino rats.
Chemicals
All reagents were of the highest quality. Zinc oxide as nanoparticles with an average size of 67 nm is a gift from Dr. Eman El-Trass. Cyclophosphamide (200 mg/ampoule) was purchased from Sigma-Aldrich.
Animals
24 male rats [Sprague Dawley, BW (150 ± 30 g)] were obtained from the faculty of agriculture, Alexandria University, and acclimatized for 2 weeks. Animals were maintained under temperature (25oC), fed with standard food, and had free access to water. Animals were divided into 4 groups and housed in galvanized wire cages. All animal procedures were performed in accordance with the Ethics Committee of Alexandria University and in accordance with the recommendations for the proper care and use of laboratory animals (NIH publication No. 85–23, revised 2007).
Experimental design
Adult male rats were divided into 4 groups (6 rats of each) as follows:
Group I. (Control group): Received saline (0.2 ml/day) i.p. injection for 14 days (day by day).
Group II. (nZnO group): Received zinc oxide nanoparticles (nZnO) (5 mg/kg/day) b.w., intraperitoneally for 14 days [20].
Group III. CP group: Received CP 20 mg/kg/day body weight (b.w.) (i.p. injection of saline) day by day for 14 days by intraperitoneal injection [21].
Group IV. CP + nZnO group: Received nZnO (5 mg/kg/day) b.w., i.p. injection for 14 days, plus CP (20 mg/kg/day) b.w., day by day for 14 days by intraperitoneal injection.
After 24-hr from the last treatments all the animals were anesthetized using light ether. Blood samples were taken from the heart within 1 min. 3 ml of blood was collected in glass tubes for coagulation and serum formation, blood was allowed to sit for 30 minutes at 4oC to clot, then centrifuged for 5 minutes at 1000 x g. The serum samples were stored into capped sterile polyethylene tubes at -20oC until used (within 24 hours). The abdominal cavity of each rat was opened where the liver and lung were excised.
Biochemical analysis
Reduced glutathione was determined according to Moron, et al. [22]. Glutathione reductase was determined spectrophotometrically according to the method of Goldberg and Spooner [23]. Catalase was determined according to Goth [24]. TBARS are expressed in terms of malondialdehyde (MDA) equivalents using the molar absorbtivity of 149000 M-1 cm-1 [25]. After animals were fasted for 24 hours of the end of the experiment, they were sacrificed. The abdominal cavity opened and lungs and liver were removed, washed with cold phosphate buffer (0.1 M, pH 7.4), weighed, and chilled on ice. A 33% crude homogenate (W/V) was prepared in phosphate buffer (0.1 M & pH 7.4) by homogenization with a Teflon pestle, using 5 strokes. The homogenate was centrifuged at 11,000 x g (4°C) for 20 minutes to remove the nuclei, mitochondria, and intact cells. The supernatant solution was subsequently centrifuged at 105,000 x g (4°C) for 60 min to sediment the microsomal pellet. The pellet was re-suspended in phosphate buffer (0.1 M, pH 7.4), and kept in an ice bath and used as the enzyme source. Liver microsomal cytochrome b5 and P450 were estimated according to Omura and Sato [26]. The activity of liver microsomal reduced nicotinamide adenine dinucleotide phosphate - cytochrome-C reductase was assayed according to the method of Williams and Kamin [27]. GST activity was estimated according to Habig, et al. [28].
Statistical analysis
The data are expressed as mean ± SE. The results were computed statistically using the SPSS software package, version 25 using one- way analysis of variance (ANOVA). Post hoc testing was performed for inter-group comparison on using the LSD. p < 0.05 was considered as significant [29].
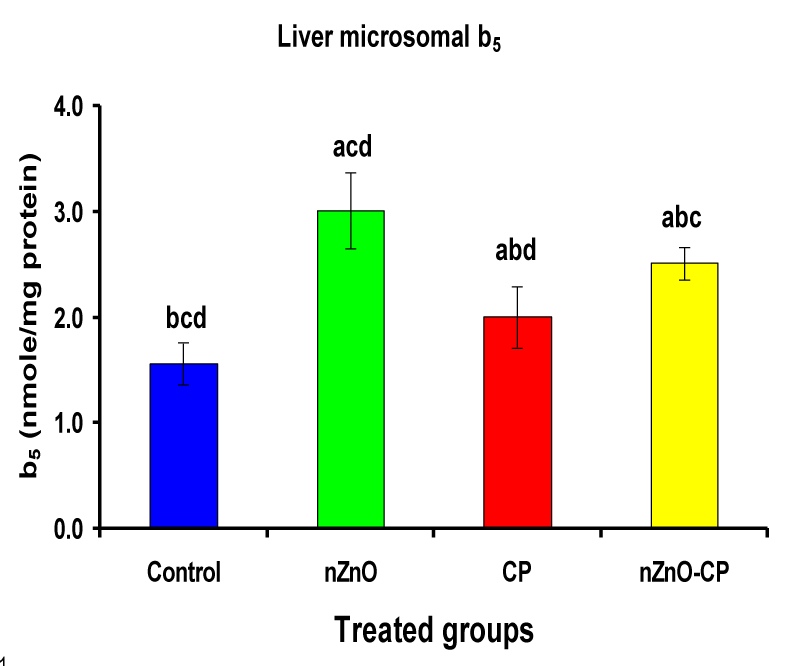
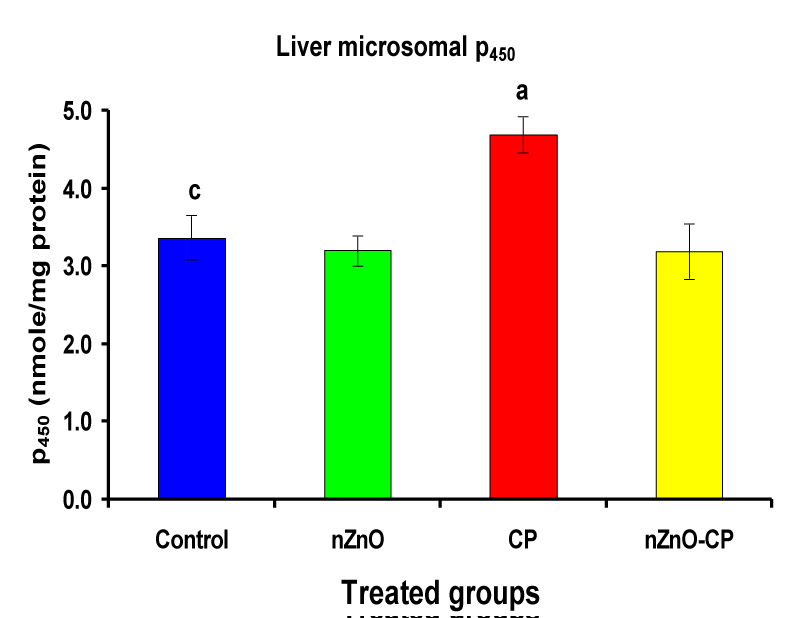
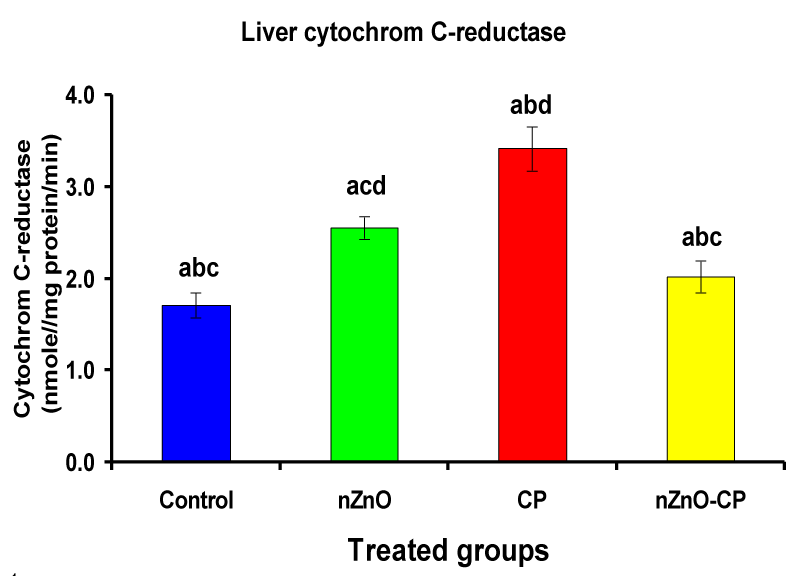
Effect of zinc oxide nanoparticles, cyclophosphamide and their combination rat liver microsomal b5, P450 and cytochrome C-reductase
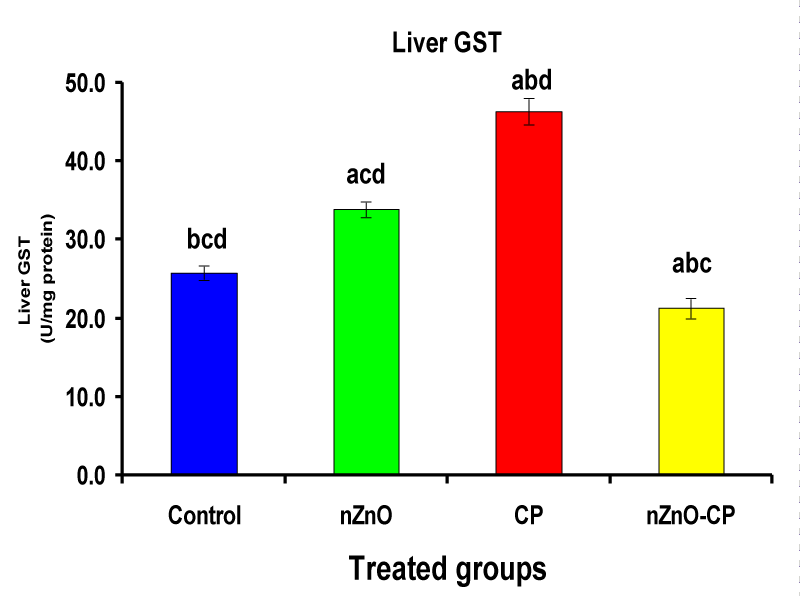
Monooxygenases are hepatic drug-metabolizing enzymes, which contain including phase I enzymes (cytochrome b5, cytochrome p450, and reduced nicotinamide adenine dinucleotide phosphate-cytochrome C reductase) which metabolize most xenobiotics and carcinogens and in phase II, GST, GSH and TBARS [30]. Individual treatment of zinc oxide nanoparticles and CP induced cytochrome b5, cytochrome C reductase, and GST compared to the control group, while CP increased P450. The combination of nZnO and CP prevents the elevation of cytochrome P450, b5, GST, and cytochrome C reductase as compared with the CP treated group (Tables 1 and Figures 1-4). Cytochrome P450 enzyme system is necessary for the bio-activation of insecticides [31,32]. Oxidative stress due to ZnNPs and CP may be ascribed to induction of Cytochrome P450, inhibition of AChE, and disturbance in contents of GSH causing lipid peroxidation [33,34].
| Table 1: Effects of treatment of rats with zinc oxide and/or cyclophosphamide on liver microsomal b5, P450, and cytochrome C-reductase. | ||||
| Groups | Control | nZnO | CP | nZnO + CP |
| Parameters | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Liver microsomal b5 (nmole/mg protein) |
1.56 ± 0.20bcd | 3.01 ± 0.36acd | 2.00 ± 0.29abd | 2.51 ± 0.15abc |
| Liver microsomal P450 (nmole/mg protein) |
3.37 ± 0.29c | 3.20 ± 0.19 | 4.69 ± 0.23a | 3.19 ± 0.36 |
| Liver microsomal cytochrome C-reductase (nmole cytochrome C-reductase/mg protein/min) | 1.71 ± 0.13bcd | 2.55 ± 0.12acd | 3.41 ± 0.24abd | 2.02 ± 0.17abc |
| Liver GST (U/mg protein) | 25.79 ± 0.9bcd | 33.85 ± 1.03acd | 46.33 ± 1.69abd | 21.30 ± 1.3abc |
| Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different. | ||||
Figure 1: Liver microsomal b5 (nmole/mg protein) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Figure 2: Liver microsomal P450 (nmole/mg protein) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Figure 3: Liver microsomal cytochrome C-reductase (nmole cytochrome C-reductase/mg protein/min) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Figure 4: Liver GST (U/mg protein) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Reduced nicotinamide adenine dinucleotide phosphate-cytochrome C reductase activity is a component of the microsomal monooxygenase which important in the metabolism of drugs, lipids, and other foreign compounds [35]. The limiting step in the detoxification and activation of toxic substances is dependent on the rate of reduction of cytochrome P450 substrate complex, which in turn is dependant on the activation and turnover rates of reduced nicotinamide adenine dinucleotide cytochrome C-reductase, cytochrome P450 and cytochrome b5 [36]. The changes produced by nanoparticles and chemotherapy treatments to reduced nicotinamide adenine dinucleotide cytochrome C-reductase activity is one of the defense mechanisms to increase the rate of reduction of cytochrome P450 substrate complex [37]. In the present study, the treatment of rats with nZnO and CP revealed a significant liver damage as observed from the elevation of hepatospecific enzyme activities. These findings demonstrate that in vivo sub-acute administration of nZnO with CP antagonize the effects of CP and modulates the activities of drug-metabolizing enzymes in rat liver, suggesting that reactive oxygen species may be involved in the toxic effects of the CP through modulation of cytochrome P450 and b5.
Effect of zinc oxide nanoparticles, cyclophosphamide and their combination rat liver homogenate thiobarbituric acid reactive substances
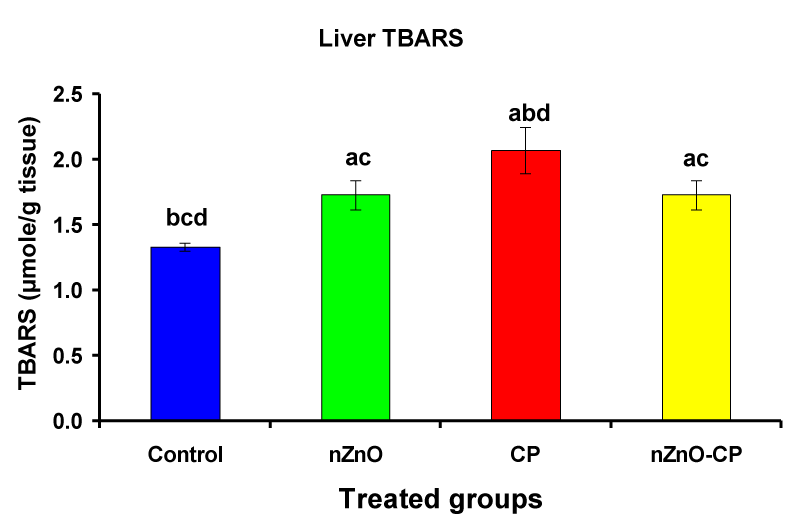
Previous studies recorded that the administration of CP leads to an increase in lipid peroxidation and a decrease in the activities of antioxidant enzymes in serum, lung, and liver of rats and mice [38-43]. Individual treatment of zinc oxide nanoparticles and CP increased (p < 0.05) liver homogenate TBARS compared to the control group, while the combination of nZnO and CP prevents the elevation of TBARS compared with the CP treated group (Table 2 and Figure 5). Lipid peroxidation (LPO) is used as a biomarker to show the index of oxidative stress, and causes of cell membranes damage, increased permeability to ions, and decreased membrane potential [44]. Induction of lipid peroxidation has been reported in different tissues of experimental animals after CP administration [39,45-47]. CP and its metabolite acrolein cause inactivation of microsomal enzymes and result in increased lipid peroxidation and ROS generation [48,49]. Co-administration of nZnO with CP caused a significant decrease in thiobarbituric acid reactive substances levels, which may be due to the antagonistic effect of this combination and/or the free radical scavenging potential of nZnO.
| Table 2: Effects of treatment of rats with zinc oxide and/or cyclophosphamide on liver Liver TBARS (µmole/g tissue). | ||||
| Groups | Control | nZnO | CP | nZnO + CP |
| Parameters | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Liver TBARS (µmole/g tissue) | 1.33 ± 0.03bcd | 1.73 ± 0.11ac | 2.07 ± 0.18abd | 1.73 ± 0.11ac |
| Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different. | ||||
Figure 5: Liver TBARS (µmole/g tissue) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Effects of zinc oxide nanoparticles, cyclophosphamide and their combination on rat serum catalase and glutathione reductase
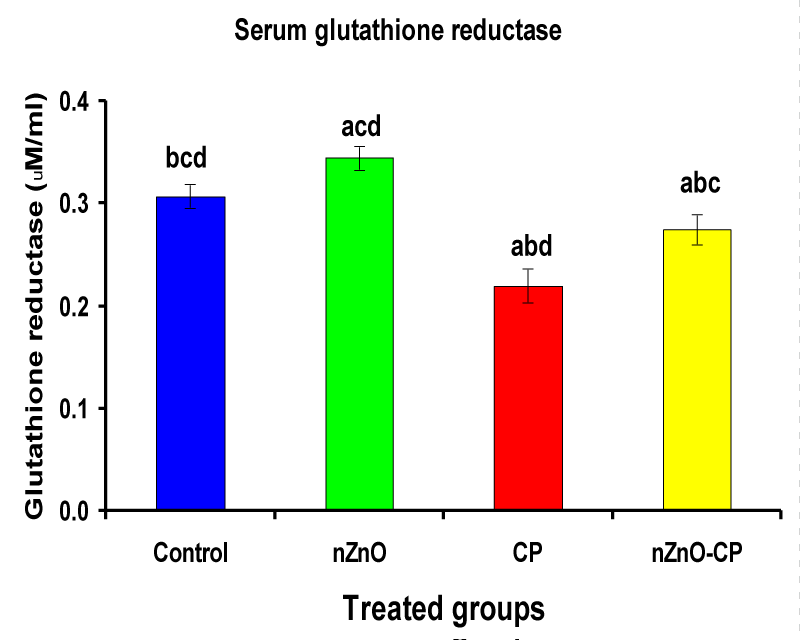
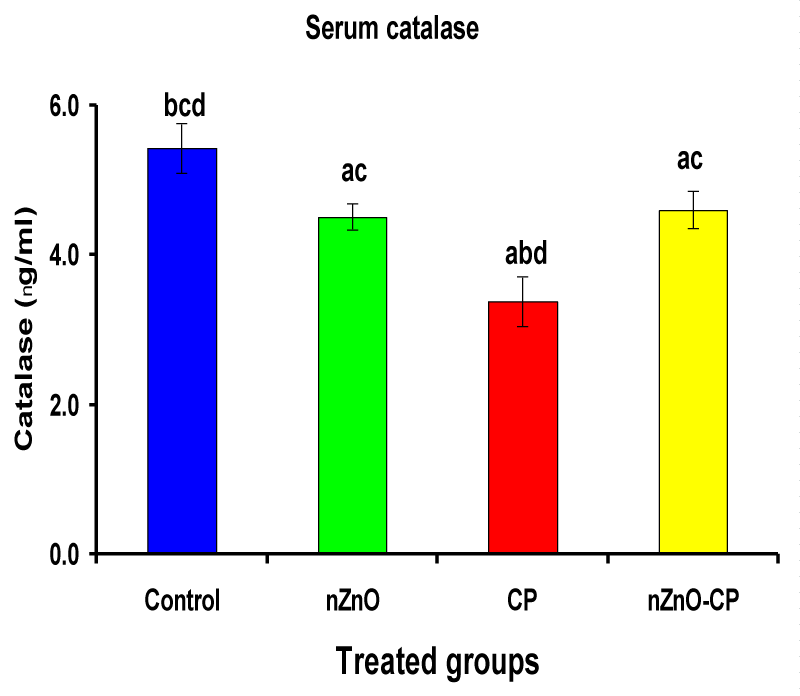
Hydroxyl radical anion, hydrogen peroxide, and superoxide are important mediators of tissue injury and DNA damage [50]. Glutathione reductase and catalase are the antioxidant enzymes that play an important role in cell defense against free radical damage. Glutathione reductase also, is an important enzyme regenerates GSH by converting GSSG. Catalase is important in the detoxification of H2O2 concentrations [51]. Injection of CP to rats reduced serum GR and CAT activities compared to control. However, combination treatment of rats with nZnO and CP increased the activities of these enzymes compared with those treated with CP alone (Table 3, Figures 6,7). These data showed that the antioxidant defense mechanisms take part in the toxicity of cyclophosphamide [52]. Durken, et al. [53] have shown that patients receiving high-dose cyclophosphamide displayed significant reductions of antioxidant parameters in plasma. In the present study many changes in rat blood serum anti-oxidative systems also have been observed after CP administration. The CP injection leads to a decrease in the activity of CAT and GR, which may be due to protein structure modification by acrolein and/or ROS generated during CP metabolism. In the present study, a significant increase in the activities of catalase and glutathione reductase and a decrease in CP-treated rats as reported earlier [47,51], which may be due to an inactivation of cellular antioxidants by the reactive oxygen species and lipid peroxides due to CP intoxication. Co-administration of nZnO with CP decreased the formation of lipid peroxidation and restored the enzyme levels, i.e. Zinc oxide nanoparticles improved the antioxidant activity, and enhanced the activities of antioxidases and decreased the levels of free radicals [54]. These improvements in the antioxidant defense system may be attributed to the antioxidant properties and/or free radical scavenging capacity of Zinc oxide nanoparticles.
| Table 3: Effects of treatment of rats with zinc oxide and/or cyclophosphamide on serum glutathione reductase (µM/ml) and cytochrome C-reductase. | ||||
| Groups | Control | nZnO | CP | nZnO + CP |
| Parameters | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Serum glutathione reductase (µM/ml) | 0.31 ± 0.01bcd | 0.34 ± 0.01acd | 0.22 ± 0.02abd | 0.27 ± 0.01abc |
| Serum catalase (ng/ml) | 5.43 ± 0.33bcd | 4.51 ± 0.18ac | 3.38 ± 0.33abd | 4.60 ± 0.25ac |
| Significance at p < 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different. | ||||
Figure 6: Serum glutathione reductase (µM/ml) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
Figure 7: Serum catalase (ng/ml) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
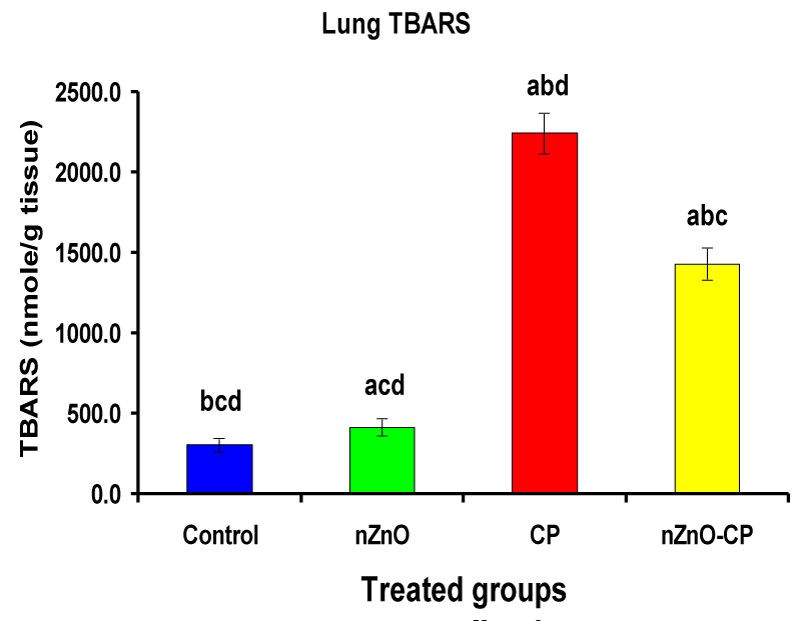
Figure 8: Lung TBARS (nM/g tissue) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
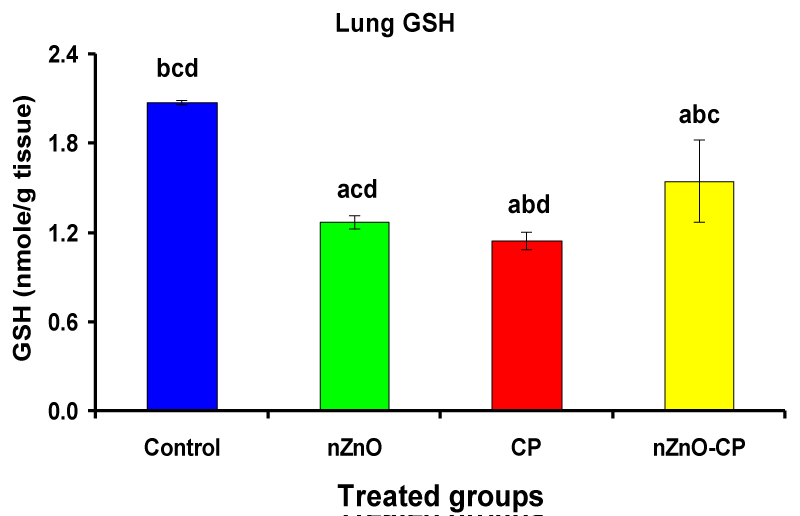
Effects of cyclophosphamide, nZnO, and their combination on rat lung thiobarbituric reactive substances and glutathione (GSH) concentration
Individual treatment of zinc oxide nanoparticles and CP increased (p < 0.05) lung homogenate TBARS compared to the control group, while the combination of nZnO and CP prevents the elevation of TBARS compared with the CP treated group (Table 4 and Figure 8). Co-administration of nZnO with CP caused a significant decrease in thiobarbituric reactive substances levels, which may be due to the antagonistic effect of this combination and/or the free radical scavenging potential of nZnO. Glutathione is an intracellular non-enzymatic antioxidant, detoxify the endogenous and exogenous toxic substances, including xenobiotics and free radicals [55]. Individual treatment of zinc oxide nanoparticles and CP decreased (p < 0.05) lung homogenate GSH compared to the control group, while the combination of nZnO and CP prevents the reduction in TBARS compared with the CP treated group (Table 4 and Figure 9). Treatment of patients with CP caused a decrease in a highly reactive electrophiles and glutathione, which may be due to the electrophilic burden on the cells and the formation of acrolein, which leads to a decrease in glutathione content [56]. Treatment of rats with nZnO increased glutathione levels in the lung glutathione catalyzes the conjugation of glutathione with a highly reactive electrophiles, which plays a major role in the detoxification of alkylating agents [57].
| Table 4: Effects of treatment of rats with zinc oxide and/or cyclophosphamide on Lung TBARS (nM/g tissue) and Lung GSH (nM/g tissue). | ||||
| Groups | Control | nZnO | CP | nZnO + CP |
| Parameters | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE |
| Lung TBARS (nM/g tissue) | 304 ± 45.3bcd | 416 ± 55.9acd | 2245 ± 127abd | 1431 ± 102abc |
| Lung GSH (nM/g tissue) | 2.08 ± 0.01bcd | 1.27 ± 0.05acd | 1.15 ± 0.06abd | 1.55 ± 0.28\abc |
| Significance at p 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different. | ||||
Figure 9: Lung GSH (nM/g tissue) of rat treated with nZnO, CP, and their combination. Significance at p > 0.05; Zinc oxide nanoparticles (nZnO); Cyclophosphamide: (CP). a Comparison of control and other groups, b Comparison of nZnO and other groups, c Comparison of CP and other groups, d Comparison of nZnO + CP and other groups, Means having the same letters are not significantly different.
It can be concluded that CP induced oxidative stress and disturbance in the antioxidant defense system. Treatment of rats with zinc oxide nano-particles and CP together attenuated the oxidative damage and disturbance in the antioxidant defense system induced by CP. So, Patients treated with CP advised to take nZnO to prevent the side effects of chemotherapy. Further studies are necessary to evaluate the amelioration effect nZnO and other nano-particles against oxidative stress induced by CP in different doses and experimental models.
- Dollery A. Cyclophosphamide. In: Dollery C., Editor. Therapeutic drugs. Edinburgh: Churchill Livingstone; 1999. 349-353.
- Nafees S, Rashid S, Ali N, Hasan SK, Sultana S. Rutin ameliorates cyclophosphamide-induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chemico-Biological Interactions. 2015; 231: 98-107. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25753322
- Lindley CM, Hamilton G, McCune JS, Faucette S, Shord SS, et al. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metabol Dispo, 2002; 30: 814-821. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12065440
- Pass GJ, Carrie D, Boylan M, Lorimore S, Wright E, et al. Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: stidies with the hepatic cytochrome P450 reductase null mouse. Canc Res. 2005; 65: 4211-4217. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15899812
- Seis H. Oxidative stress: introductory remarks. H Seis (Ed). Oxidative Stress, Academic Press, London. 1985; 1-8.
- Sachidanandam 1K, Fagan SC, Ergul A. Oxidative stress and cardiovascular disease: antioxidants and unresolved issues. Cardiovasc Drug Rev. 2005; 23: 115-132. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16007229
- Borchi E, Bargelli V, Stillitano F, Giordano C, Sebastiani M, et al. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta, 2010; 1802: 331-338. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19892017
- Schacter LP, DelVillano BC, Gordon EM, Klein BL. Red cell superoxide dismutase and sickle cell anemia symptom severity. Am J Hematol. 1985; 19: 137-144. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4003385
- Scott MD, Eaton JW, Kuypers FA, Chiu DT, Lubin BH. Enhancement of erythrocyte superoxide dismutase activity: effects on cellular oxidant defence. Blood. 1989; 74: 2542-2549. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2553167
- Kao MPC, Ang DSC, Pall A, Struthers AD. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J Human Hypertension, 2010; 24: 1-8. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19727125
- Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008; 279: 71-76. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18081843
- Wang SQ, Tooley IR. Photoprotection in the era of nanotechnology. Semin Cutan Med Surg. 2011; 30: 210-213. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22123418
- Khalaf AA, Hassanen EI, Azouz RA, Zaki AR, Ibrahim MA, et al. Ameliorative effect of zinc oxide nanoparticles against dermal toxicity induced by lead oxide in rats. Int J Nanomed. 2019; 14: 7729-7741. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31806958
- Hussein SA, EL-Senosi YA, El-Dawy K, Baz HA. Protective effect of zinc oxide nanoparticles on oxidative stress in experimental-induced diabetes in rats. Benha Veter Med J. 2014; 27: 405-414.
- Afifi M, abdelazim AM. Ameliorative effect of zinc oxide and silver nanoparticles on antioxidant system in the brain of diabetic rats. Asian Pac J Trop Biomed. 2015; 5: 874-877.
- Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008; 1: 15-24. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19794904
- El-Maddawy ZK, abd El Naby WSH. Protective effects of zinc oxide nanoparticles against doxorubicin induced testicular toxicity and DNA damage in male rats. Toxicol Res. 2019; 8: 654-662. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31588342
- Raajshreer K, Durairaj B. Evaluation of the antityrosinase and antioxidant potential of zinc oxide nanoparticles synthesized from the brown seaweed –Turbinaria conoides. Int. J Appl Pharm. 2017; 9: 116-120.
- Dawei AI, Zhisheng W, Angu Z. Protective effects of nano-ZnO on the primary culture mice intestinal epithelial cells in in vitro against oxidative injury. Int J Nanotechnol App. 2009; 3: 1-6.
- Badkoobeh P, Parivar K, Kalantar SM, Hosseini SD, Salabat A. Effect of nano-zinc oxide on doxorubicin- induced oxidative stress and sperm disorders in adult male Wistar rats. Iran J Reprod Med. 2013; 11: 355-364. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24639766
- Fahmey MA, Hassan NHA, El-Fiky SA, Elalfy HG. A mixture of honey bee products ameliorates the genotoxic side effects of cyclophosphamide. Asian Pacific J Trop Dis. 2015; 5: 638-644.
- Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979; 582: 67-78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/760819
- Goldberg DM, Spooner RJ. In HV Bergmeyer (Ed). Methods of enzymatic analysis (3rd ed). 1983; 258-265.
- Góth L. A simple method for determination of serum catalase activity and revision of reference range. Clinica Chimica Acta. 1991; 196: 143-151. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2029780
- Slater TF. Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogeno-alkanes on peroxidative reactions in rats. Biochem J. 1971; 123: 805-814. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4399399
- Omura T, Sato R. The carbon monoxide binding pigment of liver microsomes. 1-Evidence for its hematoprotein nature. J Biol Chem. 1964; 239: 1370-2378.
- Williams CH, Kamin H. Microsomal triphosphopyridine nucleotide-cytochrome-C reductase of liver. J Biol Chem. 1962; 237: 587-595.
- Habig W, Pabst M, Jakoby W. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249: 7130-139. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4436300
- Howell DC. Fundamental statistics for the behavioral sciences (3rd ed). Duxbury press. An imprint of Wads Worth publishing company Belmont. California. 1995; 163-166.
- De Oliveira CA, Gremano PM. Aflatoxins current concepts on mechanisms of toxicity and their involvement in the etiology of hepatocellular carcinoma. Rev. Saude Publica. 1997; 31: 417-424. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9595771
- Verma RS, Mehta A, Srivastava N. Effect of phenobarbitone on cytochrome P450 activity and chlorpyrifos and 3, 5, 6-trichloropyridinol levels in liver and serum in rat. Indian J Biochem Biophys. 2005; 42: 254-257. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23923552
- Goel A, Dani V, Dhawan D. Zinc mediates normalization of hepatic drug metabolizing enzymes in chlorpyrifos-induced toxicity. Toxicol Lett. 2007; 169: 26-33. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17194553
- Sharma Y, Bashir S, Irshad M, Nag TC, Dogra TD. Dimethoate-induced effects on antioxidant status of liver and brain of rats following subchronic exposure. Toxicol. 2005; 215: 173-181. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16112789
- Sivapiriya V, Jayanthisakthisekaran, Venkatraman S. Effects of dimethoate (O,O-dimethyl S-methyl carbamoyl methyl phosphorodithioate) and Ethanol in antioxidant status of liver and kidney of experimental mice. Pest Biochem Physiol. 2006; 85: 115-121.
- Vermilion JL, Ballou DP, Massey V, Coon MJ. Separate roles of FMN and FAD in catalysis by liver microsomal NADPH cytochrome P450 reductase. J Biol Chem. 1981; 256: 266-277. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/6778861
- Delvi RR. Alterations in hepatic phase I and phase II biotransformation enzymes by garlic oil in rats. Toxicol Lett. 1992; 60: 299-305. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1595088
- Sheweita S, abd El-Gaber M, Bastawy M. Carbon tetrachloride changes the activity of cytochrome P450 system in the liver of male rats: role of antioxidants. Toxicol. 2001; 169: 83-92. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11718950
- Patel JM, Block ER. Cyclophosphamide-induced depression of the antioxidant defense mechanisms of the lungs. Exp Lung Res. 1985; 8: 153-165. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4029094
- Venkatesan N, Chandrakasan G. Modulation of cyclophosphamide induced early lung injury by curcumin, an anti-inflammatory antioxidant. Mol Cell Biochem. 1995; 142: 79-87. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7753045
- Kaya H, Oral B, Ozguner F, Tahan V, Babar Y, et al. The effect of melatonin application on lipid peroxidation during cyclophosphamide therapy in female rats. Zentralbl Gynakol. 1999; 121: 499-502. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10573825
- Lear L, Nation RL, Stupans I. Effects of cyclophosphamide and adriamycin on rat hepatic microsomal glucuronidation and lipid peroxidation. Biochem Pharmacol. 1992; 44: 747-753. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/1510722
- Mathew S, Kuttan G. Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. J Exp Clin Cancer Res. 1997; 16: 407-411. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9505214
- Premkumar K, Pachiappan A, Abraham SK, Santhiya ST, Gopinath PM. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia. 2001; 72: 906-911. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11731115
- Halliwell B, Gutteridge. JM. Free radicals in biology and medicine, 2nd ed. Oxford: Clarendon Press. 1989.
- Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, et al. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum Exp Toxicol. 2003; 22: 473-480. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14580007
- Patel JM. Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicol. 1987; 45: 79-91. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/3603576
- Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-alpha-lipoic-acid. Mol Cell Biochem. 2005; 272: 179-185. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16010986
- Adams JD, Klaidman LK. Acrolein induced oxygen radical formation. Free Radic Biol Med. 1993; 15: 187-193. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8397144
- Uchida K. Current status of acrolein as a lipid product. Trends Cardiovasc Med. 1999; 9: 109-113. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10639724
- Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993; 23: 21-48. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8471159
- Lin HM. Yen FL, Ng LT, Lin CC. Protective eff ects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J Ethnopharmacol. 2007; 111: 129-136.
- Parke DV, Sapota A. Chemical toxicity and reactive oxygen species. Int J Occup Med Env Health. 1996; 9: 331. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/9117192
- Durken M, Agbenu J, Finckh B, Hubner C, Pichlmeier U, et al. Deteriorating free radical-trapping capacity and antioxidant status in plasma during bone marrow transplantation. Bonne Marrow Transplant. 1995; 15: 7570. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7670403
- Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012; 745: 84-91. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22198329
- Ali-Osman F. Quenching of DNA cross-link precursors of chloroethylnitrosoureas and attenuation of DNA interstrand cross-linking by glutathione. Cancer Res. 1989; 49: 5258-5260. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2766294
- McDiarmid MA, Iype PT, Koldner K, Jacobnson KD, Strickland PT. Evidence for acrolein-modified DNA in peripheral blood leucocytes of cancer patients treated with cyclophosphamide. Mutat Res. 1991; 248: 93-99. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2030715
- Touliatos JS, Neitzel L, Whitworth C, Rybak LP, Malafa M. Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat. Eur Arch Otorhinolaryngol. 2000; 25: 6-9. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10664037