More Information
Submitted: October 09, 2024 | Approved: October 29, 2024 | Published: October 30, 2024
How to cite this article: Murdock DK. Pharmacological Manipulation of the Aging Pathways to Effect Health Span and Lifespan with Special Reference to SGLT2 Inhibitors as Powerful Anti-aging Agents in Humans. Insights Biol Med. 2024; 8(1): 011-025. Available from: https://dx.doi.org/10.29328/journal.ibm.1001028
DOI: 10.29328/journal.ibm.1001028
Copyright License: © 2024 Murdock DK. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aging; Chronic diseases of aging; SGLT2 inhibitors
Pharmacological Manipulation of the Aging Pathways to Effect Health Span and Lifespan with Special Reference to SGLT2 Inhibitors as Powerful Anti-aging Agents in Humans
David K Murdock*
The Department of Cardiology, Aspirus Wausau Hospital and the Aspirus Research Institute, USA
*Address for Correspondence: David K Murdock, The Department of Cardiology, Aspirus Wausau Hospital and the Aspirus Research Institute, USA, Email: [email protected]; [email protected]
Calorie restriction has been shown to slow the aging process in numerous organisms including primates. Caloric excess states, such as type 2 diabetes, are associated with accelerated aging and the incidence and severity of chronic diseases. The nutrient-sensing pathways and intestinal microbiome are important systems that affect aging and chronic disease development. This manuscript reviews the various pathways involved with aging and chronic disease development and examines the pharmacological manipulation of these systems which appear to slow aging and the chronic diseases of aging in experimental model organisms and collaborating human data when available. Finally, the abundance of experimental and human data suggesting the newer diabetic medications, the sodium-glucose transport inhibitors, are potent anti-aging agents is provided.
Aging is the most common disease affecting mankind and ultimately has a 100% mortality rate. Most age-related mortality is due to one or more of the chronic diseases aging causes and/or the frailty associated with aging. Modern theories of aging fall into two main categories: the genetically programmed theory and the accumulation of damage-causing genetic error theory [1]. The programmed theory suggests aging follows a preprogrammed genetically determined biological timetable similar to mechanisms dictating childhood growth and development. The damaging error theory postulates aging results from an accumulation of environmental and chemical insults unavoidably occurring from cellular metabolism and existing in an oxidizing and hostile environment. Both theories ultimately recognize changes in gene expression occur that effect the systems responsible for maintenance, repair, and defense responses. These changes can be observed and characterized on the cellular and molecular level and are now recognized as the 9 “Hallmarks of Aging”. These hallmarks include genomic instability, epigenetic alterations, telomere attrition, and the accumulation of proteotoxins due to loss of proteostasis. This leads to deregulated nutrient sensing, mitochondrial dysfunction, and cellular senescence causing stem cell exhaustion and altered intercellular communication ultimately inducing the functional decline and development of the chronic diseases associated with aging [2]. Since age is the basis for most chronic diseases, therapeutic interventions targeting aging could have huge consequences on health span and the cost of providing health care. This manuscript reviews the pathways that influence aging and chronic disease development and highlights some of the pharmacological means used to affect these pathways leading to a slowing of aging and chronic disease development. Additionally, the manuscript makes the case that the newer diabetic agents, the sodium-glucose transporters inhibitors, are effective antiaging agents with an abundance of human data to support that conclusion.
The effect of calorie restriction and calorie excess on aging and chronic diseases
In the early 1900s, it was discovered that dietary manipulations could affect health and longevity of select organisms. Reduction of food intake and dietary changes decreased the occurrence of cancers in rodents [3] and increased the lifespan in rats [4] and fruit flies [5]. In 1933 McKay, et al. [6] demonstrated that the increase in the life span of mice by dietary manipulation was due to Calorie Restriction (CR). These observations effectively demonstrated that life span, health span, and aging were not fixed and could be modifiable by behavior and its epigenetic effects. Subsequent studies confirmed this observation and extended it to multiple other species including primates [7-12]. Epidemiologic data strongly suggests that CR also slows aging and prevents chronic disease in humans. The inhabitants of Okinawa are a good example of this phenomenon. Their low-calorie plant-based diet is associated with both a long-life span as well as a marked reduction in the chronic diseases of aging [13].
In leu of the benefits seen with CR, it is not surprising that caloric excess states may have the opposite effect on aging and the development of chronic diseases. Higher caloric diets have been shown to reduce the life span of C. elegans [14] and mice [15]. Additionally, human data also suggest a strong correlation between obesity, accelerated aging, life span, and chronic diseases [16,17]. Type 2 diabetes is a classic exam of a caloric excess metabolic state. Type 2 diabetes is now recognized as a disease of accelerated aging [18,19]. Additionally, the chronic diseases of aging are seen more often and occur earlier in type 2 diabetic patients [19,20]. Consequently, investigations of interventions that may have gero-protective effects might manifest this protection earlier and more decisively in the diabetic population.
The nutrient-sensing pathway and their effect on aging
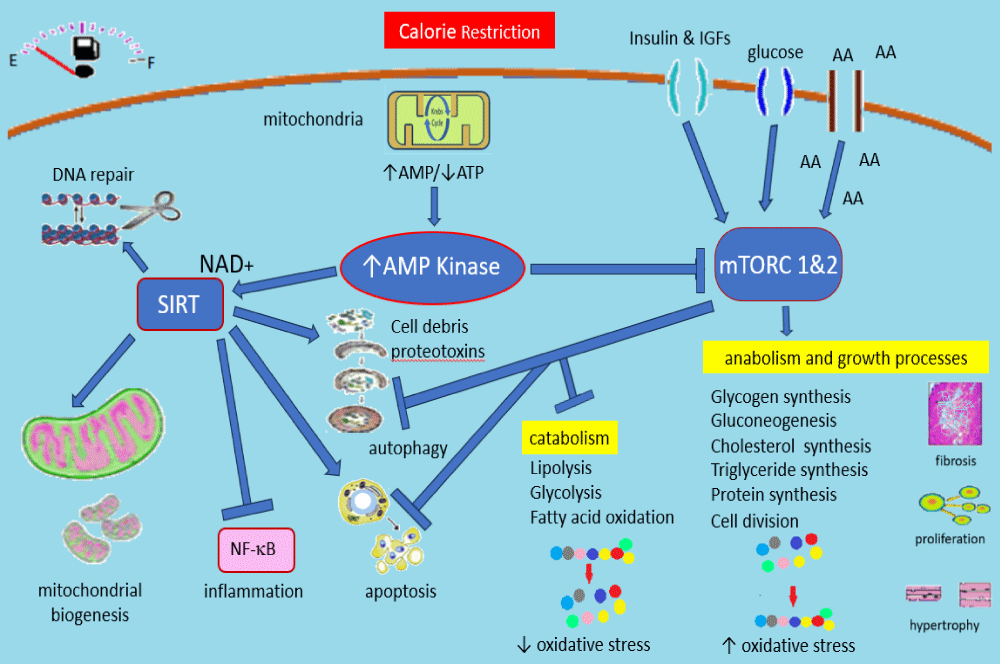
Recent advances have significantly extended our knowledge of the molecular and epigenetic mechanisms that mediate lifespan extension by CR. CR works through the key nutrient signaling pathways including the Insulin/insulin-like growth factor (IGF) signaling pathway (IIS), mammalian target of rapamycin (mTOR), AMP Kinase, and sirtuins [21]. CR downregulates the IIS pathway and mTOR pathway while activating AMP kinase and the sirtuin pathways. Direct effects, as well as cross-talks between these pathways, lead to a cascade of events whereby growth and other anabolic pathways are suppressed, and catabolic processes are stimulated (Figure 1).
Figure 1: Summarizes many of the biological effects occurring during calorie restriction. The down regulation of mTOR due to falling insulin and IGF levels as well as the inhibitory effects of AMP kinase activation leads to a switch from anabolic to catabolic processes. Sirtuin activation occurs and the net effect is a marked decrease in oxidative damage, inflammation, proteotoxins, and activation of the cellular clean-up mechanisms including genomic repair, apoptosis, and autophagy. These cellular repair and preservation measures are felt to be instrumental in the slowing of aging and the occurrence of chronic disease processes observed with CR. See text for details. AA: amino acids, IGFs: insulin-like growth factors, NF-B: nuclear factor kappa B.
Insulin and insulin-like growth factors (IGFs) are the first line of defense against nutrient deprivation. Insulin responds predominately to circulating glucose levels but also responds to amino acid and fatty acid levels [22]. IGFs also respond to nutrient levels, predominately protein consumption [23]. Insulin and IGF are strong anabolic hormones that mediate much of their effect by activating the mTOR pathway [24]. Low insulin and IGF-1 levels are associated with longevity as are loss of function mutations of the IGF pathway [25,26]. High levels are associated with accelerated aging and more rapid development of chronic diseases [27].
mTOR serves as a master controller of numerous metabolic processes and responds both directly to nutrient signals, such as amino acid levels, as well as interacting with other nutrient sensing systems; the insulin signaling pathways and AMP Kinase [24,28,29]. mTOR is a protein kinase that forms the catalytic subunit of two distinct protein complexes, known as mTOR Complex 1 (mTORC1) and 2 (mTORC2) [28]. In order to grow and divide, cells must increase anabolic pathways which include the production of complex carbohydrates, proteins, lipids, and nucleotides while at the same time suppressing catabolic pathways, such as autophagy and apoptosis. mTORC1 plays a major role in regulating these processes and therefore controls the balance between anabolism and catabolism in response to nutrient availability. mTORC2 is intimately involved in cellular proliferation, the production of the microtubules forming the cellular cytoskeleton, and the regulation of apoptosis [29]. While mTORC1 is particularly sensitive to energy substrate availability; such as amino acids, mTORC2 is particularly simulated by hormonal growth factors; such as insulin and IGFs [29]. A reduction in the activity of mTOR pathways is strongly implicated as a major cause of the effect of CR on the aging of diverse organisms including yeast, worms, flies, and mammals by decreasing inflammation and oxidative stress, the accumulation of proteotoxins, altered immunity and increasing in the cellular repair mechanisms [30].
AMP kinase responds to AMP and ATP levels and is activated in response to relative energy deficits brought about by CR or exercise [28]. AMP kinase activation inhibits mTOR and thus favors activation of the cellular protective response pathways while down-regulating the anabolic growth and proliferative pathways [31]. AMP kinase also facilitates the sirtuin pathways [32].
The mammalian sirtuins (SIRT1–SIRT7) are an energy-sensing system controlling numerous cellular protective mechanisms including DNA repair and genome stability, mitochondrial biogenesis, autophagy, and inflammation [33,34]. This is primarily a histone deacetylation system that works via epigenetic regulation and uses NAD+ as a cofactor. CR increases NAD+ levels and activates sirtuins. Certain sirtuins have ADP-ribosyltransferase activity and are particularly important for genomic stability [35]. SIRT1 is found in most organisms, and its ortholog SIR2 found in yeast has been the most studied thus far. Genetic manipulation leading to overexpression of SIR2 in yeast [36] or SIRT1 in C. elegans [37] increases the life span of these organisms while deletion of SIRT 1 has the opposite effect [37]. These observations pave the way for pharmacologic manipulation of these pathways as a possible means to slow aging.
The role of beta hydroxybutyrate
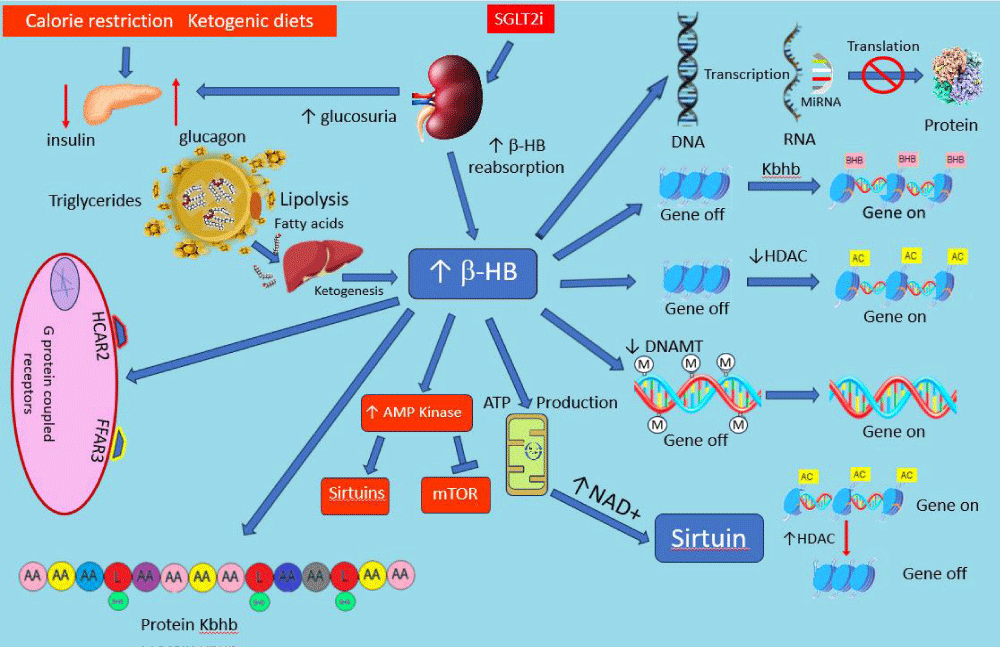
The decrease in the insulin-to-glucagon ratio, the activation of AMP kinase, and the decrease in mTOR activity associated with CR leads to a catabolic state with enhanced lipolysis, fatty acid utilization, and ketogenesis. β-hydroxybutyrate (β-HB) is the most abundant ketone and in addition to serving as an efficient energy source plays an important role in the anti-aging effects of CR. Dietary β-HB supplementation, or a non-CR ketogenic diet, has been shown to extend life and health span and promote the anti-aging phenotype in numerous organisms [38-41]. A non-CR ketogenic diet has also been shown to positively affect several chronic diseases of aging including cancer, cardiovascular disease, and neurodegenerative diseases [38,41]. It is now clear that β-HB is far more than an alternative energy source. β-HB exerts a multitude of biological effects by serving as a signaling molecule via several different pathways summarized in Figure 2. β-HB can activate AMP kinase independent of the effects due to CR [40,42] and thus mimics the biological consequences of CR depicted in Figure 1. As an energy substrate, the metabolism of β-HB is relatively NAD+ sparing, making more of this cofactor available to activate the sirtuin system [43]. β-HB also produces several direct epigenetic effects via DNA methylation and histone modification. β-HB interferes with DNA methylation lessening the oppressive effects of DNA methylation on transcription [44,45]. β-HB is an endogenous inhibitor of histone deacetylation (HDAC) [45-47]. Since HDAC generally leads to gene silencing, the inhibition of HDAC increases gene expression. Finally, a unique form of histone modification occurs when β-HB binds to lysine in some histones (lysine β-hydroxybutyrylation usually abbreviated as Kbhb) promoting active gene transcription [45-47].
Figure 2: There are multiple pathways in which -HB effects cellular metabolic processes. Several epigenetic effects via multiple different mechanisms exist (right side of figure). -HB can directly activate AMP kinase which in turn facilitates the sirtuin system and inhibits mTOR affecting the biological processes depicted in Figure 1. -HB can both activate genetic transcription in some pathways and decrease activity in others ultimately effecting hundreds of genes and the proteins they code for. In addition to epigenetic effects, -HB is also a ligand for at least two cell-surface G-protein-coupled receptors HCAR2 and FFAR3. Finally, -HB can modify histone and non-histone proteins by a process called Lysine β-hydroxybutyrylation (Kbhb) which can affect the activity of the proteins. Ultimately these effects lead to profound metabolic changes promoting an antiaging effect. See text for details. AA: amino acid, AC: Acetylation, -HB: beta hydroxybutyrate, DNAMT: DNA methyl transferase, FFAR3: free fatty acid receptor 3, HCAR2: hydroxycarboxylic acid receptor 2, HDAC: histone de-acetylation, Kbhb: Lysine β-hydroxybutyrylation, L: lysine, MET: Methylation, MiRNA: micro-RNA, mTOR: mammalian target of rapamycin, SGLT2I: sodium-glucose transport inhibitor.
MicroRNAs (miRNAs) are small non-coding RNAs consisting of about 20–22 nucleotides formed during the processing of longer RNA transcripts. MicroRNA exerts epigenetic effects by binding to target messenger RNA to control their translation into proteins [48]. A ketogenic diet has been shown to influence microRNA activity involved in cytokine signaling pathways, and antioxidant and anti-inflammatory signaling pathways reducing the damaging effects of these pathways [49]. Taken together, it is clear that β-HB can both up-regulate and down-regulate transcription depending upon when and where it intervenes.
Just as β-HB can bind to lysine and modify histone proteins to effect transcription, it can also bind to lysine in some non-histone proteins modifying their function by changing the physical and spatial configuration of the protein. This in turn affects its chemical properties and activity; a process called protein Kbhb [50-52]. Although the study of protein Kbhb is relatively new, and the totality of its effect is still to be determined, it is now clear that hundreds of different proteins have been identified undergoing modification by Kbhb [52].
Finally, in addition to its genomic and proteomic effects, β-HB is also a ligand for at least two cell-surface G-protein-coupled receptors, the hydroxycarboxylic acid receptor 2 (HCAR2) and the free fatty acid receptor 3 (FFAR3). The binding of β-HB to HCAR2 reduces atherosclerosis and inflammation while FFAR3 binding effects both inflammation and lipid metabolism [46,51].
In summary, it is now increasingly clear that β-HB exerts a multitude of biologic effects via numerous mechanisms leading to a beneficial effect on several of the “Hallmarks of Aging” [53]. These effects most likely explain the gero-protective affects observed with β-HB supplementation and the ketogenic diet in the experimental setting. Pharmacological manipulation of β-HB levels could prove to be a useful method to effect health span and life span in humans. However, at this time, the data to support this remains limited.
The role of the microbiome on chronic diseases and aging
The intestinal microbiome refers to the trillions of microorganisms that colonize the GI tract and includes bacteria, fungi, archaea, viruses, and protozoans [54,55]. It is enormously diverse with over 1000 taxa typically present though the phyla Firmicutes, Actinobacteria, and Bacteroidetes constitute the vast majority of the biomass [55]. The diversity protects the overgrowth of pathogenic organisms. For the most part these organisms as a whole form a symbiotic relationship with the host and help regulate the immune system, protect against other pathogenic bacteria that cause disease, and produce vitamins including vitamin K and several B vitamins [55]. The microbiome is especially useful to help break down complex carbohydrates and dietary fibers producing short-chain fatty acids (SCFA) such as acetate, propionate, and butyrate [56]. These SCFAs feed the gut lining cells, decrease inflammation and help keep our overall gut environment healthy [56,57]. SCFA production also inhibits the growth of pathogenic organisms by reducing luminal pH. The lower pH decreases the production of phenolic compounds, and ammonia and decreases the activity of undesirable bacterial enzymes [56,57].
Genetic sequencing studies, (metagenomics) has greatly increased our understanding of the microbiome. Metagenomics has revealed the diversity of the microbiome is much larger than that revealed by cultures and is highly variable from individual to individual depending upon environmental factors, such as diet, use of alcohol, activity level, geographical location, medications, as well as host genetic factors and age [54,58,59].
Interest in the biome greatly accelerated when it was discovered the microbiome was different in patients with a range of disorders compared with healthy individuals [54,60-63]. A change in the makeup of the microbiota composition, such that it becomes deleterious to host health, is termed “dysbiosis”. Dysbiosis has been implicated in numerous disorders, ranging from luminal disorders, such as colon-rectal cancers and inflammatory bowel diseases, to disorders resulting from more systemic effects such as coronary artery disease, diabetes, obesity, hypertension, neural degenerative diseases, arthritis, chronic kidney disease, respiratory illnesses and to immune-mediated inflammation [54,60-64].
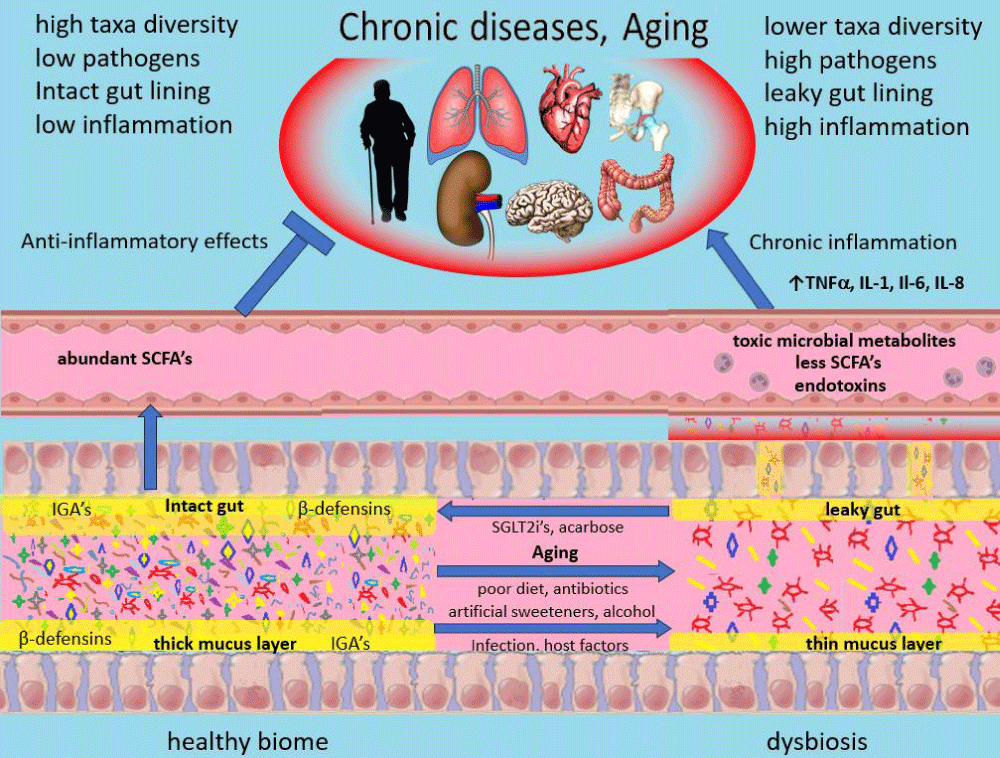
Much research has been undertaken to understand how dysbiosis produces widespread systemic effects on health. Dysbiosis is not a single specific entity but exists in multiple forms with different species involved. In its most severe forms, dysbiosis can be its own clinical entity. Cholera and toxigenic diarrheas serve as examples [65]. More commonly dysbiosis exists as a subclinical condition characterized by a decrease in overall diversity of the microbiome, an increase in pro-inflammatory species, and a decrease in helpful anti-inflammatory species [60-64]. Several beneficial metabolites produced by a healthy microbiome are produced less robustly. SCFAs for instance are less abundant [62-64]. SCFAs not only exert local beneficial mucosal effects but also enter the circulation and exert beneficial anti-inflammatory effects on numerous other organs via interaction with G protein-coupled receptors as well as by epigenetic mechanisms due to inhibition of histone deacetylase [64,66]. Net proinflammatory effects on the intestinal mucosa and toxic microbial metabolites damage the mucosa cells and this damage along with altered mucus production impairs gut barrier function causing a “leaky gut” [63,67]. The migration of bacteria and endotoxin into the submucosal region activates the immune system [63,66,67]. The leakage of toxic microbial metabolites and endotoxins into the circulation exports inflammation systemically via various inflammatory cytokines and interleukins [63,66,67]. This chronic low-grade inflammation is felt to be instrumental in how dysbiosis contributes to the multiple chronic diseases of aging previously mentioned [54,60-64] (Figure 3).
Figure 3: The healthy intestinal biome consists of a large diversity of organisms and an abundance of healthy bacteria illustrated here as multiple colors and shapes within the lumen. These healthy bacteria secrete various metabolic substances (SCFAs, polyamines, and many others) which help keep pathogens in check and contribute to the health of the mucosal lining. The mucus membrane also helps deter bacterial invasion as do antibacterial IgA antibodies and -defensins. With dysbiosis, there is a significant decrease in diversity and a relative increase in pathogens. Fewer SCFAs and other protective metabolites are produced. An increase in toxic bacterial metabolites may lead to damaged luminal epithelia cells and decreased production of mucus. This can lead to a “leaky gut” allowing bacteria to cross the luminal membrane releasing toxic metabolites and causing low-grade endotoxemia. This incites an innate immune response with the production of proinflammatory cytokines. This inflammation, along with toxic metabolites produced by pathogens, can cause damage locally but also leads to accelerated damage in numerous other organs and ultimately accelerated aging. See text for additional details. IgA: Immunoglobulin A, IL: interleukin, SCFA: Short chain fatty acids, TNF: tumor necrosis factor alpha.
The finding that dysbiosis is linked to many of the chronic diseases associated with aging raises the intriguing possibility that it may also be involved with aging itself. The biome typically undergoes dramatic changes with age which are typical of dysbiosis [68-71]. The changes are so common with age that dysbiosis and the associated low levels of inflammation which it produces, termed “inflammaging”, are now recognized as additional “Hallmarks of Aging” [71]. In support of a biome effect upon aging, healthy very old people (centenarians) typically do not demonstrate dysbiosis and have one with a greater potential for SCFA production and less pathogenic taxa [68-70].
Further support for the microbiome affecting aging comes from elegant heterochronic Fecal Microbiota Transplantation (FMT) studies using various experimental model organisms [72-75]. Using mice, standardized techniques were used to perform metagenomic sequencing and assess proteomics while cognitive effects were assessed using a battery of cognitive and behavioral tests. FMT from aged mice into young mice caused CNS changes resulting in impaired spatial learning and memory disorders in young adult recipients similar to that present in non-transplanted elderly mice [71,72]. Aged donor microbiota transferred into young mice drove inflammation and loss of integrity in the intestinal mucosal barrier resulting in elevated systemic and tissue markers of inflammation and upregulated inflammation in the retina and brain [72]. Equally fascinating was FMT from young donors into elderly recipients. This process reversed the effects normal aging had on cognition and markers of inflammation in elderly mice [73]. Not only has FMT effected the aging phenotype but various experimental models have shown the ability of heterochronic FMT to slow aging and prolong life span. The African turquoise killifish is a short-lived fish whose microbiota changes significantly as it ages [74]. While young fish harbor highly diverse microbial communities, older fish have less diverse microbiota and more microbes associated with disease. FMT from young fish into middle age fish significantly increased their life span and improved the diversity of their microbiome [74]. Finally using an accelerated aging progeroid mouse model, which like human progeroids develops dysbiosis, FMT from healthy wild-type mice enhanced health span and lifespan [75].
The enormous amount of data associating dysbiosis with health span, chronic diseases, and aging has stimulated extensive research into ways to modify the microbiome and reverse the effects of dysbiosis. Probiotics are living microbes that when ingested effect the microbiome either by colonizing it with favorable microbes or exerting positive effects by influencing the existing microbiome (non-colonizing effects). As far back as the early 1900’s it was observed that modifying the gut microbiome by replacing harmful bacteria with more favorable ones by consuming fermented products containing Lactobacillus bulgaricus was associated with longevity and health [76]. These early observations of the value of probiotics have been greatly expanded upon in recent years. Probiotics have been shown to have significant effects on gut permeability by increasing mucin production, enhancing β-defensin expression and secretion into the mucus by epithelial cells, promoting secretion of antibacterial IgA into the luminal mucous layer, and some probiotics can directly kill or inhibit the growth of pathogenic bacteria via expression of antimicrobial bacteriocins [77].
Experimental studies on the benefits of various probiotics on aging have yielded promising results in several models. The probiotic Bacillus subtilis produced a significant extension of lifespan in Caenorhabditis elegans [78]. A species of gut bacteria called Bifidobacterium adolescentis decreases with age in humans [79]. Dietary supplementation of B. adolescentis improved osteoporosis and neurodegeneration in a mouse model of premature aging, and increased health span and lifespan in Drosophila melanogaster and C. elegans [79].
Although human studies of the effect of probiotics on aging and life span are limited there is intriguing data that such effects could well exist. In a recent metanalysis of 42 randomized clinical trials using various cocktails of microorganisms, probiotic supplementation in humans significantly reduced serum concentrations of several of the pro-inflammatory cytokines; hs-CRP, TNF-a, IL-6, IL-12, and IL-4, but it did not influence IL-1B, IL-8, IFN-g, and IL-17 [80]. The same study found a significant increase in serum concentrations of IL-10, an anti-inflammatory cytokine [80]. Since “inflammaging” is considered one of the hallmarks of aging [71], these beneficial effects could very likely slow aging.
Another approach to a healthier microbiome is to use prebiotics which are carbohydrate compounds, primarily oligosaccharides, known to resist digestion in the human small intestine and reach the colon where they are fermented by the gut microflora [81,82]. The fermentation of these carbohydrates represents a major source of energy for epithelial cells with the production of SCFAs. Prebiotics provide a beneficial physiological effect by stimulating the growth and/or activity of beneficial bacterial species in the gut [81,82]. Lactobacilli and bifidobacteria are the usual target genera for prebiotics. These prebiotics are commonly referred to as fiber, the health benefits of which are well establish [83]. High-fiber vegetables, legumes, whole grains, and fruits are examples of natural prebiotics. Commercially prepared prebiotics generally contain fiber from various sources including inulin, oligofructose, fructo-oligosaccharides, galactose-containing and xylose-containing oligosaccharides, lactulose, and polydextrose [84]. Short-term human studies have shown that the microbiome can be favorably altered by prebiotic supplementation [85,86]. Whether this will ultimately lead to health benefits will require longer-term studies which have yet to be completed.
In summary, the discovery of the importance of the microbiome’s contribution to our health challenges the very concept of what we are as humans. We are symbiotes dependent on trillions of primitive organisms in order to survive. With better delineation and cost-effective ways to assess the presence of dysbiosis using metagenomic sequencing, and improved capabilities to favorably alter the microbiome, it is possible that assessment of the microbiome will become a regular part of health maintenance, just as other periodic measurements are routinely preformed to monitor health.
Pharmacological manipulation of the pathways involved in aging
Although CR has been shown to prolong life and health span, applying it to humans has been challenging. The CALERIE 2 Study attempted to impose a 25% calorie restriction on participants [87]. Unfortunately, compliance was not as planned. The average CR during the first 6 months was 19.5 ± 0.8% and 9.1 ± 0.7% over the next 18 months of the study. Never-the-less, even this smaller reduction in calories is associated with improved cardiometabolic risk factors. Whether this would be sufficient to slow aging in humans remains unknown.
The recognition that CR slows aging and improves the health span has sparked enormous interest in attempting to reproduce the health benefits of CR by pharmacologic means. Biohacking the nutrient-sensing pathways and microbiome has proven to be a useful means to accomplish this goal in a variety of species. The following is a brief review of some of the research demonstrating the potential benefits of biohacking the nutrient-sensing pathways by mimicking the effects of CR.
The IIS pathway: Acarbose is a complex oligosaccharide used in the treatment of type 2 diabetes that acts as a competitive inhibitor of intestinal alpha-glucosidase. By delaying the digestion of carbohydrates, acarbose slows glucose absorption, resulting in a reduction of postprandial glucose and insulin levels in patients with and without diabetes [88]. In the experimental heterogenous mouse model, acarbose suppressed IGF-1 and insulin and increased the health span and life span of aging mice [89]. The decrease in IGF-1 and insulin levels with acarbose would in turn downregulate the IIS pathway and its effect on mTOR (Figure1). Human studies with acarbose have demonstrated a marked decrease in inflammatory cytokines (IL-6, TNF-α, IL-1β) with chronic administration in diabetic patients [90]. In the Beijing Community Diabetes Study where urban Chinese diabetic patients were followed over 10 years, acarbose was associated with a significant reduction in cardiovascular death and all-cause mortality [91]. Finally, in addition to its effect on the IIS/mTOR pathways, acarbose has been shown to alter the microbiome in humans [92] and mice [93], with an increase in the production of SCFAs.
Recognizing the importance of the effects of IGF-1/mTOR signaling on aging [25,26] and the fact that large dogs with a short lifespan have much higher IGF-1 levels than smaller longer living dogs [94,95], Loyal, a clinical-stage veterinary medicine company, has developed an RNA antisense agent (LOY-001) to inhibit IGF-1 [95]. The FDA has agreed that this agent could plausibly increase the lifespan and health span of large dogs and is in the process of reviewing this agent for veterinarians’ use [95]. This agent may become the first drug approved by the FDA for the sole purpose of slowing aging and prolonging the health and life span of a mammalian species.
mTOR pathway: Rapamycin, discovered on the Island or Rapa Neu and named after the Island (known to most as Easter Island), was found to inhibit a major energy sensing complex which was subsequently named after it; the mechanistic target of rapamycin or mTOR (Figure1 [96]). Direct inhibition of mTOR by rapamycin has proven to be remarkably successful in slowing aging. Rapamycin and other mTOR inhibiting agents have been shown to prolong lifespan and health span in mice [97,98] as well as numerous other species [99-101] by switching metabolic processes to favor the cellular protective mechanisms. Many other mTOR inhibitors are being explored as possible antiaging agents.
AMP kinase: Activation of AMP kinase inhibits mTOR and activates the sirtuin system (Figure 1). This favors the activation of the cellular protective response pathways. Metformin is a medication commonly used to treat type 2 diabetes. It is a weak inhibitor of ATP production which consequently activates AMP kinase and has been shown to increase life span and health span in mice [102,103]. In a recent proof-of-concept study involving male cynomolgus monkeys of 13 to 16 years (approximately equivalent to 40–50 years in humans), metformin produced a significant slowing of aging indicators, notably a roughly 6-year regression in brain aging as measured by slowing cognitive decline and brain atrophy [104]. Similar antiaging effects were noted in several other organs as well. Finally, there is intriguing human data that metformin has gero-protective effects in diabetic humans by reducing all-cause mortality and chronic diseases of aging, particularly cardiovascular disease, and cancers [105]. Additionally, metformin was shown to favorably effect the human gut microbiome by favoring the proliferation of bacteria associated with anti-inflammatory effects and with SCFA production [106]. Numerous other direct and indirect activators of AMP kinase exist from multiple chemical classes [107]. Most have not been investigated with regard to the effects of aging.
Sirtuins: Sirtuins interact with all the major conserved longevity pathways; AMP-Kinase, insulin/IGF-1 signaling (IIS), and mTOR (Figure 1 [108]). The search for molecules that activate sirtuins has yielded several promising results. These sirtuin-activating compounds (STACs) are mainly divided into two categories; exogenous activators which allosterically bind to the sirtuin increasing their activity, such as resveratrol [108,109], to compounds that replenish cellular NAD+, a necessary cofactor for sirtuin activity [110]. These NAD+ precursors include nicotinamide (NAM), nicotinic acid (NA), nicotinamide riboside (NR), and nicotinamide mononucleotide (NMN) [110].
The first STACs were discovered in 2003 for sirt1 and its yeast ortholog SIR2 [109]. Resveratrol, a polyphenol, proved to be the most potent STAC [109]. This agent prolonged the lifespan of yeast by 70%. Subsequent studies have shown resveratrol given to mice attenuated age-related and degenerative changes in multiple organs and reduced inflammation without an appreciable effect on life span [111]. Since these early observations high-throughput screening techniques have identified more than 14,000 STACs from multiple chemical classes [109].
NAD+ precursor supplementation using NR and NMN appears to restore NAD+ levels in both nuclear and mitochondrial compartments of multiple cell types [110,112]. Numerous murine models have shown attenuation of the aging phenotype with various NAD+ precursor supplementation protocols [113,114]. As of today, there remains a paucity of human data showing the benefits of NAD+ precursor supplementation or the use of other STACs [115].
Despite the abundance of animal data showing the feasibility of prolonging the life and health span in model organisms by pharmacologic means, other than metformin and acarbose, there is little data yet available in humans to support this approach. Studies using life span as the end point to gauge success in humans would be lengthy and enormously costly to perform. However, since health span and incidence of chronic disease are greatly influenced by aging, looking at the effects of possible age-slowing agents on the incidence and severity of chronic diseases and all-cause mortality in large, randomized populations over specific time periods is a feasible way to evaluate this possibility. Indeed, it is this type of data that suggests metformin and acarbose have gero-protective effects as evidenced by a decrease in all-cause mortality and chronic diseases of aging in humans [92,106].
The case for SGLT2 inhibitors as gero-protective agents
The gliflozins are a group of compounds containing a glucose moiety attached to various aromatic rings. These agents can occupy, but not effectively transverse, the sodium and glucose transport (SGLT) systems in numerous tissues. SGLT type 2 is abundant in renal tissue and inhibition of these transporters by competitive occupation results in enhanced renal sodium and glucose excretion [116]. These glucosuria effects are responsible for their usefulness in controlling the glucose abnormalities of type 2 diabetes.
The effect of SGLT2 inhibitors on the nutrient-sensing pathways, β-HB, and the microbiome
SGLT2 inhibitors (SGLT2Is) affect directly or indirectly all the key nutrient-sensing pathways involved with aging [117]. The glycosuria induced by this inhibition produces a loss of about 200-300 kcal/day consistently causing a mild weight loss [118]. Moreover, the loss of glucose as a substrate is associated with a shift toward ketogenesis and fat utilization due to decreased insulin and increased glucagon levels; like what is seen with CR [119,120]. As a consequence, significant increases in β-HB levels are seen in both diabetic and non-diabetic patients [121,122]. As insulin is one of many activators of mTOR, a reduction in insulin levels will have an indirect inhibitory effect on mTOR [24]. Additionally, the caloric drag produced by SGLT2Is as well as direct effects on mitochondrial ATP production activates AMP kinase [117,123] producing potent inhibitory effects on mTOR [24,117]. Since AMP kinase also activates the sirtuin system, the caloric drag produced by SGLT2Is has been shown to activate the sirtuin system even in tissue devoid of SGLT2 proteins [117,124]. This combined effect on the nutrient-sensing pathways and β-HB thus mimics that seen with CR and like CR, the activity of hundreds if not thousands of different genes is affected.
Like metformin and acarbose, SGLT2Is has been shown to affect the microbiome. In a randomized trial in diabetics with risk factors for CVD after a 3-month course of empagliflozin improved glucose metabolism and reduced CVD-related risks and inflammatory markers, while it significantly altered the gut microbiota, including an increase in SCFA-producing bacteria and a reduction in several harmful bacteria such as Escherichia–Shigella, Bilophila, and Hungatella [125]. Other studies have also shown similar increases in SCFA-producing bacteria as well as an improvement in intestinal permeability [126].
The combined effects of SGLT2Is on the nutrient-sensing pathways, ketogenesis and β-HB levels, and the microbiome likely account for the effects seen on several of the hallmarks of aging observed in human and animal models. These effects include a reduction in cellular senescence and inflammation [127,128], reduced mitochondrial dysfunction and oxidative stress [129], enhanced autophagy [130], improved nutrient sensing [131], improved proteostasis [132], and reduced stem cell exhaustion [133]. Though not specifically studied yet, due to the attenuation of inflammation and oxidative stress [127-129] it is likely these agents would improve genomic stability and reduce telomere attrition [134,135].
The effect of SGLT2 inhibition on chronic diseases of aging
Given the effect of SGLT2Is on the nutrient-sensing pathways, β-HB, and the microbiome it is interesting to examine the human data on the effect of these agents on some of the chronic diseases of aging. Among the most common diseases of aging causing morbidity and mortality in developed countries are cardiovascular disease, hypertension, chronic kidney disease, chronic obstructive pulmonary disease, neurodegenerative disease, cancer, and type 2 diabetes. Evidence is emerging that suggests SGLT2Is may attenuate each of these chronic diseases.
Cardiovascular disease
The necessary cardiovascular outcome trials to gain FDA approval yielded exciting and unexpected cardiac protective effects in diabetic patients with atherosclerotic cardiovascular disease (ASCVD) and congestive heart failure (CHF) subjected to SGLT2Is. For example, SGLT2I empagliflozin produced a 38% decrease in cardiovascular mortality in diabetic patients who had preexisting ASCVD [136]. Similar but somewhat less impressive benefits were seen with other SGLT2Is [137,138]. Subsequent studies confirmed these agents also have marked protective effects in patients with CHF due to LV dysfunction and in those with preserved LV function [139-142]. The observed reduction in mortality and hospitalizations was seen both in diabetic and non-diabetic patients and was not felt to be related to glucose control.
The aging heart is characterized by an increase in left ventricular mass due to hypertrophy and interstitial fibrosis resulting in diastolic dysfunction, loss of myocytes due to apoptosis and necrosis, and reduced ability to generate energy owing to mitochondrial dysfunction [143-145]. These same effects occur at a more rapid pace in patients with CHF and this adverse cardiac remodeling contributes to the onset and progression of heart failure severity [146]. Aging also results in stiffening of the great vessels due to increased collagen and decreased elastin [145]. The myocardial and vascular changes occur in a milieu of increased proinflammatory cytokines which are instrumental in driving the process [145-148].
Several experimental and human studies have demonstrated the beneficial effects of SGLT2Is on cardiac remodeling and vascular physiology. Structural and functional changes include attenuation and/or reversal of adverse remodeling in animal models of CHF [149] and regression of LV mass in human CHF patients with a reduction in cardiac fibrosis leading to improved diastolic function [150-152]. Also, improved energy metabolism with decreased oxidative stress occurs [151,152]. These beneficial effects extend to the great vessels whereby SGLT2Is attenuate fibrotic activity reducing the stiffness of the great vessels and improving endothelial function [153] while significantly decreasing inflammatory cytokines [151-153]. These effects contribute to the antihypertensive effects consistently observed with SGLT2s [153].
To summarize, cardiovascular disease is the most common chronic disease of aging and the leading cause of mortality in developed countries. SGLT2Is attenuate and in some cases reverse the adverse changes associated with aging causing a dramatic clinical benefit in humans.
Chronic renal disease
Aging produces a gradual decrease in renal function associated with loss of renal mass, afferent arteriolar hyalinization, and an increase in the number of sclerotic glomeruli and tubulointerstitial fibrosis [154]. Inflammation plays a crucial role in accelerating these changes [155] The effect of SGLT2 inhibitors on chronic kidney disease has now been studied in numerous placebo-controlled trials in both diabetics and non-diabetics and has consistently shown a slowing of the progression of renal dysfunction [156-159]. CR, with its attendant effect on the nutrient-sensing pathways, has been shown to slow the progression of kidney disease in multiple rodent models [160]. There is now considerable evidence that alteration of these nutrient sensing pathways by SGLT2Is plays a crucial role in slowing the progression of chronic kidney disease in humans [156-159,161].
Other chronic diseases of aging
Although not as systemically studied as they were with the cardiovascular and renal systems, there is considerable experimental and observational clinical data suggesting SGLTIs may benefit several other chronic diseases of aging. In large retrospective cohort studies of patients with type 2 diabetes SGLT2Is use was associated with a reduced risk of developing chronic obstructive lung disease and a lower rate of exacerbations compared with dipeptidyl peptidase 4 (DPP-4) use [162,163]. There is growing evidence that SGLT2Is also have neuroprotective effects. SGLT2Is have been shown to protect against cognitive decline and adverse histological changes in a murine model of dementia [164] and a large retrospective propensity-matched cohort study has noted patients with type 2 diabetes who were prescribed SGLT2Is had a lower risk of incident dementia compared with those not prescribed SGLT2Is in real-world practice [165]. Anti-cancer benefits have been noted in several experimental studies utilizing various cancer cell lines [166] and a large meta-analysis of the randomized placebo-controlled SGLT2Is clinical trial has demonstrated a markedly lower incidence of cancer in those diabetics receiving SGLT2Is [167]. Finally, from a meta-analysis of randomized trials prediabetics receiving SGLT2Is have a lower risk of developing future type 2 diabetes [168].
Effect of SGLT2I on all-cause mortality
A gero-protective agent used in a large population over time should be associated with a reduction in all-cause mortality by decreasing mortality from chronic disease states. Several studies, including meta-analysis of controlled studies, as well as large observational database reviews have now confirmed that the SGLT2Is are effective in reducing all-cause mortality [169-173]. Moreover, this reduction in all-cause mortality was observed regardless of diabetic status or the presence or absence of CHF [172]. Additionally, when compared to DDP4 inhibitors, diabetic patients on SGLT2Is experienced significantly lower risks of all-cause death, cardiovascular death, cancer death, and non-cancer, non-vascular death [173].
Effect of SGLT2Is on life span in experimental models
Despite the abundance of clinical evidence for an antiaging effect of SGLT2Is, experimental studies testing that hypothesis are few. However, one compelling and confirmatory study was done by the Interventions Testing Program (ITP). The ITP is considered the gold standard for such studies and uses an otherwise healthy heterogenous mouse model. The tests of potentially gero-protective agents are run simultaneously in 3 separate labs. Using this model ITP recently reported canagliflozin prolonged the lifespan of male mice but had no significant effect on female mice [174]. Subsequent histopathological studies on these mice demonstrated a lower incidence and later appearance of cardiomyopathy, kidney disease, arteriosclerosis, and various neoplasms confirming both health span and lifespan benefits [175]. This slowing of the aging phenotype by histological means was confirmed in middle-aged wild-type mice fed canagliflozin beginning at 50 weeks and continued for another 20 weeks [176]. The same study showed canagliflozin also prolonged the life span of a progeria-type mouse model of accelerating aging in both sexes [176].
In summary, there is an abundance of clinic and experimental data to suggest that SGLT2Is have gero-protective properties. Available human data strongly suggest these agents increase health span by decreasing the incidence and severity of the chronic diseases of aging and favorably affect all-cause mortality.
It has been over 100 years since it was learned that the rate of aging could be modified by calorie restriction. Since then, medical science has discovered numerous ways to alter the nutrient-sensing pathways and microbiome to effect aging in model organisms. Since aging is the basis for most chronic diseases, targeting aging in humans could have huge benefits on health span and the economics of providing health care. The use of gero-protective agents to accomplish this goal in humans is still in its infancy but exciting data attesting to this possibility is beginning to emerge. This is particularly evident in the diabetic population, a population characterized by accelerated aging. There is now compelling evidence in humans that several of the anti-diabetic agents have antiaging properties working through the nutrient-sensing pathways and microbiome. This evidence is particularly impressive with the SGLT2Is and I believe represents the strongest evidence so far that pharmacological means can alter the rate of aging and chronic disease in humans.
The author recognizes limitations in this assertion. Most of the data comes from the diabetic population and it is not clear if the same robust antiaging effects of the SGLT2Is would be evident in the non-diabetic population. Encouraging data does suggest that the benefits of SGLT2is on chronic kidney disease and congestive heart failure also extend to the non-diabetic population as well. Whether other chronic diseases of aging would similarly benefit is unknown and requires additional studies. Finally testing the antiaging properties of SGLT2Is in other model organisms needs to be accomplished. If these studies are also confirmatory, the implications to the health care system could be enormous.
- J. Modern biological theories of aging. Aging Dis. 2010;1(2):72-74. Available from: https://pubmed.ncbi.nlm.nih.gov/21132086/
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-217. Available from: https://doi.org/10.1016/j.cell.2013.05.039
- Rous P. The influence of diet on transplanted and spontaneous mouse tumors. J Exp Med. 1914;20:433–451. Available from: https://doi.org/10.1084/jem.20.5.433
- Osborne TB, Mendel LB, Ferry EL. The effect of retardation of growth upon the breeding period and duration of life of rats. Science. 1917;45:294–295. Available from: https://doi.org/10.1126/science.45.1160.294
- Loeb J, Northrop JH. What determines the duration of life in metazoa? Proc Natl Acad Sci USA. 1917;3:382–386. Available from: https://doi.org/10.1073/pnas.3.5.382
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. Available from: https://doi.org/10.1093/jn/10.1.63
- Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass, and disease. J Gerontol. 1982;37:130–141. Available from: https://doi.org/10.1093/geronj/37.2.130
- Guarente L. Calorie restriction and SIR2 genes–towards a mechanism. Mech Ageing Dev. 2005;126:923–928. Available from: https://doi.org/10.1016/j.mad.2005.03.013
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mech Ageing Dev. 2005;126:938–950. Available from: https://doi.org/10.1016/j.mad.2005.03.023
- Houthoofd K, Vanfeteren JR. Longevity effect of dietary restriction in Caenorhabditis elegans. Exp Gerontol. 2006;41:1026–1032. Available from: https://doi.org/10.1016/j.exger.2006.05.007
- Ingle L, Wood TR, Banta AM. A study of longevity, growth, reproduction, and heart rate in Daphnia longispina as influenced by limitation in quantity of food. J Exp Zool. 1937;76:325–352. Available from: https://ui.adsabs.harvard.edu/link_gateway/1937JEZ....76..325I/doi:10.1002/jez.1400760206
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. Available from: https://doi.org/10.1126/science.1173635
- Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr. 2009;28:500S-516S. Available from: https://doi.org/10.1080/07315724.2009.10718117
- Franco-Juárez B, Gómez-Manzo S, Hernández-Ochoa B, Cárdenas-Rodríguez N, Arreguin-Espinosa R, Pérez de la Cruz V, et al. Effects of high dietary carbohydrate and lipid intake on the lifespan of C. elegans. Cells. 2021;10(9):2359. Available from: https://doi.org/10.3390/cells10092359
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337-342. Available from: https://doi.org/10.1038/nature05354
- Tam BT, Morais JA, Santosa S. Obesity and ageing: two sides of the same coin. Obes Rev. 2020;21(4). Available from: https://doi.org/10.1111/obr.12991
- Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all-cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:12156. Available from: https://doi.org/10.1136/bmj.i2156
- Bahour N, Cortez B, Pan H, Shah H, Doria A, Aguayo-Mazzucato C. Diabetes mellitus correlates with increased biological age as indicated by clinical biomarkers. Geroscience. 2022;44(1):415-427. Available from: https://doi.org/10.1007/s11357-021-00469-0
- Burton D, Faragher R. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19:447–459. Available from: https://doi.org/10.1007/s10522-018-9763-7
- Morley JE. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 2008;24(3):395-405. Available from: https://doi.org/10.1016/j.cger.2008.03.005
- Hwangbo DS, Lee HY, Abozaid LS, Min YJ. Mechanisms of lifespan regulation by calorie restriction and intermittent fasting in model organisms. Nutrients. 2020;12(4):1194. Available from: https://doi.org/10.3390/nu12041194
- Newsholme P, Krause M. Nutritional regulation of insulin secretion: implications for diabetes. Clin Biochem Rev. 2012;33(2):35-47. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC3387883/
- Ketelslegers JM, Maiter D, Maes M, Underwood LE, Thissen JP. Nutritional regulation of insulin-like growth factor-I. Metabolism. 1995;44(10 Suppl 4):50-57. Available from: https://doi.org/10.1016/0026-0495(95)90221-x
- Yoon MS. The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients. 2017;9(11):1176. Available from: https://doi.org/10.3390/nu9111176
- Heemst DV. Insulin, IGF-1, and longevity. Aging Dis. 2010;1(2):147-157. Available from: https://pubmed.ncbi.nlm.nih.gov/22396862/
- Altintas O, Park S, Lee S-JV. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016;49:81–92. Available from: https://doi.org/10.5483/bmbrep.2016.49.2.261
- Kolb H, Kemp K, Martin S. Insulin and aging – a disappointing relationship. Front Endocrinol. 2023;14:1261298. Available from: https://doi.org/10.3389/fendo.2023.1261298
- Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960-976. Available from: https://doi.org/10.1016/j.cell.2017.02.004
- Papadopoli D, Boulay K, Kazak L, Pollak M, Mallette F, Topisirovic I, et al. mTOR as a central regulator of lifespan and aging. F1000Res. 2019;8. Available from: https://doi.org/10.12688/f1000research.17196.1
- Stallone G, Infante B, Prisciandaro C, Grandaliano G. mTOR and aging: an old fashioned dress. Int J Mol Sci. 2019;20(11):2774. Available from: https://doi.org/10.3390/ijms20112774
- Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116(7):1776-1783. Available from: https://doi.org/10.1172/jci29044
- Sadria M, Layton AT. Interactions among mTORC, AMPK, and SIRT: a computational model for cell energy balance and metabolism. Cell Commun Signal. 2021;19:57. Available from: https://doi.org/10.1186/s12964-021-00706-1
- Kelly G. A review of the sirtuin system, its clinical implications, and the potential role of dietary activators like resveratrol: part 1. Altern Med Rev. 2010;15(3):245-263. Available from: https://pubmed.ncbi.nlm.nih.gov/21155626/
- Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007;21(8):1745–1755. Available from: https://doi.org/10.1210/me.2007-0079
- Aravind L, Zhang D, de Souza RF, Anand S, Iyer LM. The natural history of ADP-ribosyltransferases and the ADP-ribosylation system. Curr Top Microbiol Immunol. 2015;384:3-32. Available from: https://doi.org/10.1007/82_2014_414
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13(19):2570–2580. Available from: https://genesdev.cshlp.org/content/13/19/2570.short
- Dall KB, Færgeman NJ. Metabolic regulation of lifespan from a C. elegans perspective. Genes Nutr. 2019;14:25. Available from: https://genesandnutrition.biomedcentral.com/articles/10.1186/s12263-019-0650-x
- Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, et al. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab. 2017;26(3):547-557.e8. Available from: https://doi.org/10.1016/j.cmet.2017.08.004
- Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab. 2017;26(3):539-546.e5. Available from: https://doi.org/10.1016/j.cmet.2017.08.005
- Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC. D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging (Albany NY). 2014;6(8):621-644. Available from: https://doi.org/10.18632/aging.100683
- Han YM, Ramprasath T, Zou MH. β-Hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. 2020;52:548–555. Available from: https://doi.org/10.1038/s12276-020-0415-z
- Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, et al. β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444-66454. Available from: https://doi.org/10.18632/oncotarget.12119
- Gómora-García JC, Montiel T, Hüttenrauch M, Salcido-Gómez A, García-Velázquez L, Ramiro-Cortés Y, et al. Effect of the ketone body, D-β-hydroxybutyrate, on Sirtuin2-mediated regulation of mitochondrial quality control and the autophagy-lysosomal pathway. Cells. 2023;12(3):486. Available from: https://doi.org/10.3390/cells12030486
- Allison J, Kaliszewska A, Uceda S, Reiriz M, Arias N. Targeting DNA methylation in the adult brain through diet. Nutrients. 2021;13(11):3979. Available from: https://doi.org/10.3390/nu13113979
- Ungaro P, Nettore IC, Franchini F, Palatucci G, Muscogiuri G, Colao A, et al. Epigenome modulation induced by ketogenic diets. Nutrients. 2022;14(15):3245. Available from: https://doi.org/10.3390/nu14153245
- Wang L, Chen P, Xiao W. β-Hydroxybutyrate as an anti-aging metabolite. Nutrients. 2021 Sep 28;13(10):3420. Available from: https://doi.org/10.3390/nu13103420
- Newman JC, Verdin E. β-Hydroxybutyrate: a signaling metabolite. Annu Rev Nutr. 2017 Aug 21;37:51-76. Available from: https://doi.org/10.1146/annurev-nutr-071816-064916
- Bartel DP. Metazoan microRNAs. Cell. 2018;173(1):20–51. Available from: https://doi.org/10.1016/j.cell.2018.03.006
- Cannataro R, Caroleo MC, Fazio A, La Torre C, Plastina P, Gallelli L, Lauria G, Cione E. Ketogenic diet and microRNAs linked to antioxidant biochemical homeostasis. Antioxidants (Basel). 2019;8(8):269. Available from: https://doi.org/10.3390/antiox8080269
- Hou W, Liu G, Ren X, Liu X, He L, Huang H. Quantitative proteomics analysis expands the roles of lysine β-hydroxybutyrylation pathway in response to environmental β-hydroxybutyrate. Oxid Med Cell Longev. 2022;2022:4592170. Available from: https://doi.org/10.1155/2022/4592170
- García-Velázquez L, Massieu L. The proteomic effects of ketone bodies: implications for proteostasis and brain proteinopathies. Front Mol Neurosci. 2023;16:1214092. Available from: https://doi.org/10.3389/fnmol.2023.1214092
- Huang H, Zhang D, Weng Y, Delaney K, Tang Z, Yan C, Qi S, Peng C, Cole PA, Zhao Y. The regulatory enzymes and protein substrates for the lysine β-hydroxybutyrylation pathway. Sci Adv. 2021;7. Available from: https://doi.org/10.1126/sciadv.abe2771
- Han YM, Ramprasath T, Zou MH. β-Hydroxybutyrate and its metabolic effects on age-associated pathology. Exp Mol Med. 2020;52(4):548-555. Available from: https://www.nature.com/articles/s12276-020-0415-z
- Ursell LK, Metcalf JL, Parfrey LW, Knight R. Defining the human microbiome. Nutr Rev. 2012;70(8):440-454. Available from: https://doi.org/10.1111/j.1753-4887.2012.00493.x
- Ho JT, Chan GC, Li JC. Systemic effects of gut microbiota and its relationship with disease and modulation. BMC Immunol. 2015;16:21. Available from: https://doi.org/10.1186/s12865-015-0083-2
- Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7(3):189-200. Available from: https://doi.org/10.1080/19490976.2015.1134082
- Kim CH. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell Mol Immunol. 2023;20:341–350. Available from: https://www.nature.com/articles/s41423-023-00963-0
- Wang WL, Xu SY, Ren ZG, Tao L, Jiang JW, Zheng SS. Application of metagenomics in the human gut microbiome. World J Gastroenterol. 2015;21(3):803-814. Available from: https://doi.org/10.3748/wjg.v21.i3.803
- Human Microbiome Project Consortium. Structure, function, and diversity of the healthy human microbiome. Nature. 2012;486:207–214. Available from: https://doi.org/10.1038/nature11234
- Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375:2369–2379. Available from: https://doi.org/10.1056/NEJMra1511570
- Walker AW, Lawley TD. Therapeutic modulation of intestinal dysbiosis. Pharmacol Res. 2013;69(1):75-86. Available from: https://doi.org/10.1016/j.phrs.2012.09.008
- Vijay A, Valdes AM. Role of the gut microbiome in chronic diseases: a narrative review. Eur J Clin Nutr. 2022;76(4):489-501. Available from: https://doi.org/10.1038/s41430-021-00991-6
- Al Samarraie A, Pichette M, Rousseau G. Role of the gut microbiome in the development of atherosclerotic cardiovascular disease. Int J Mol Sci. 2023;24(6):5420. Available from: https://doi.org/10.3390/ijms24065420
- Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016;158:52-62. Available from: https://doi.org/10.1016/j.pharmthera.2015.11.012
- Kopic S, Geibel JP. Toxin-mediated diarrhea in the 21st century: the pathophysiology of intestinal ion transport in the course of ETEC, V. cholerae, and rotavirus infection. Toxins (Basel). 2010;2(8):2132-57. Available from: https://doi.org/10.3390/toxins2082132
- Zhao M, Chu J, Feng S, Guo C, Xue B, He K, Li L. Immunological mechanisms of inflammatory diseases caused by gut microbiota dysbiosis: a review. Biomed Pharmacother. 2023;164:114985. Available from: https://doi.org/10.1016/j.biopha.2023.114985
- Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2023. Available from: https://doi.org/10.1007/s11739-023-03374-w
- Badal VD, Vaccariello ED, Murray ER, Yu KE, Knight R, Jeste DV, Nguyen TT. The gut microbiome, aging, and longevity: a systematic review. Nutrients. 2020;12(12):3759. Available from: https://doi.org/10.3390/nu12123759
- Pang S, Chen X, Lu Z, Meng L, Huang Y, Yu X, Huang L, Ye P, Chen X, Liang J, Peng T, Luo W, Wang S. Longevity of centenarians is reflected by the gut microbiome with youth-associated signatures. Nat Aging. 2023;3:436–449. Available from: https://doi.org/10.1038/s43587-023-00389-y
- Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of “healthy” aging of elderly people. Immun Ageing. 2021;18(1):2. Available from: https://doi.org/10.1186/s12979-020-00213-w
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186:243-278. Available from: https://doi.org/10.1016/j.cell.2022.11.001
- D'Amato A, Di Cesare Mannelli L, Lucarini E, Man AL, Le Gall G, Branca JJV, et al. Fecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. Available from: https://doi.org/10.1186/s40168-020-00914-w
- Parker A, Romano S, Ansorge R, Aboelnour A, Le Gall G, Savva GM, et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome. 2022;10(1):68. Available from: https://doi.org/10.1186/s40168-022-01243-w
- Smith P, Willemsen D, Popkes M, Metge F, Gandiwa E, Reichard M, Valenzano DR. Regulation of lifespan by the gut microbiota in the short-lived African turquoise killifish. eLife. 2017;6. Available from: https://doi.org/10.7554/eLife.27014
- Bárcena C, Valdés-Mas R, Mayoral P, Durand S, Rodríguez F, Fernández-García MT, et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat Med. 2019;25:1234–1242. Available from: https://doi.org/10.1038/s41591-019-0504-5
- Metchnikoff E. The prolongation of life: optimistic studies. New York, NY: GP Putnam's Sons; 1908. Translated by Mitchell PC.
- Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2010;298. Available from: https://doi.org/10.1152/ajpgi.00243.2009
- Donato V, Ayala FR, Cogliati S, Bauman C, Costa JG, Leñini C, Grau R. Bacillus subtilis biofilm extends Caenorhabditis elegans longevity through downregulation of the insulin-like signaling pathway. Nat Commun. 2017;8:14332. Available from: https://doi.org/10.1038/ncomms14332
- Chen S, Chen L, Qi Y, Xu J, Ge Q, Fan Y, et al. Bifidobacterium adolescentis regulates catalase activity and host metabolism and improves healthspan and lifespan in multiple species. Nat Aging. 2021;1:991–1001. Available from: https://doi.org/10.1038/s43587-021-00129-0
- Milajerdi A, Mousavi SM, Sadeghi A, Salari-Moghaddam A, Parohan M, Larijani B, Esmaillzadeh A. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. 2020;59(2):633-649. Available from: https://doi.org/10.1007/s00394-019-01931-8
- Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417-35. Available from: https://doi.org/10.3390/nu5041417
- Kerry RG, Patra JK, Gouda S, Park Y, Shin HS, Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927-939. Available from: https://doi.org/10.1016/j.jfda.2018.07.002
- Anderson JW, Baird P, Davis RH Jr, Ferreri S, Knudtson M, Koraym A, Waters V, Williams CL. Health benefits of dietary fiber. Nutr Rev. 2009;67(4):188-205. Available from: https://doi.org/10.1111/j.1753-4887.2009.00189.x
- Patel S, Goyal A. The current trends and future perspectives of prebiotics research: a review. 3 Biotech. 2012;2:115–125. Available from: https://link.springer.com/article/10.1007/s13205-012-0044-x
- Healey G, Murphy R, Butts C, Brough L, Whelan K, Coad J. Habitual dietary fibre intake influences gut microbiota response to an inulin-type fructan prebiotic: a randomized, double-blind, placebo-controlled, cross-over, human intervention study. Br J Nutr. 2018;119(2):176-189. Available from: https://doi.org/10.1017/S0007114517003440
- Mitchell CM, Davy BM, Ponder MA, McMillan RP, Hughes MD, Hulver MW, Neilson AP, Davy KP. Prebiotic inulin supplementation and peripheral insulin sensitivity in adults at elevated risk for type 2 diabetes: a pilot randomized controlled trial. Nutrients. 2021;13(9):3235. Available from: https://doi.org/10.3390/nu13093235
- Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, et al. A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol A Biol Sci Med Sci. 2015;70(9):1097-104. Available from: https://doi.org/10.1093/gerona/glv057
- Alssema M, Ruijgrok C, Blaak EE, Egli L, Dussort P, Vinoy S, et al. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: a systematic review and meta-analysis. Nutr Diabetes. 2021;11:11. Available from: https://www.nature.com/articles/s41387-021-00152-5
- Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-alpha-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. Available from: https://doi.org/10.1111/acel.12170
- Mo D, Liu S, Ma H, Tian H, Yu H, Zhang X, et al. Effects of acarbose and metformin on the inflammatory state in newly diagnosed type 2 diabetes patients: a one-year randomized clinical study. Drug Des Devel Ther. 2019;13:2769-2776. Available from: https://doi.org/10.2147/DDDT.S208327
- Zhang XL, Yuan SY, Wan G, Yuan MX, Yang GR, Fu HJ, et al. The effects of acarbose therapy on reductions of myocardial infarction and all-cause death in T2DM during 10-year multifactorial interventions (The Beijing Community Diabetes Study 24). Sci Rep. 2021;11(1):4839. Available from: https://doi.org/10.1038/s41598-021-84015-0
- Zhang X, Fang Z, Zhang C, Xia H, Jie Z, Han X, et al. Effects of acarbose on the gut microbiota of prediabetic patients: A randomized, double-blind, controlled crossover trial. Diabetes Ther. 2017;8(2):293-307. Available from: https://doi.org/10.1007/s13300-017-0226-y
- Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019;19:130. Available from: https://doi.org/10.1186/s12866-019-1494-7
- Greer KA, Hughes LM, Masternak MM. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr). 2011;33(3):475-83. Available from: https://doi.org/10.1007/s11357-010-9226-3
- Loyal. Loyal announces historic FDA milestone for large dog lifespan extension drug. Available from: https://loyalfordogs.com/posts/loyal-announces-historic-fda-milestone-for-large-dog-lifespan-extension-drug
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78(1):35-43. Available from: https://doi.org/10.1016/0092-8674(94)90519-0
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends longevity of genetically heterogeneous mice. Nature. 2009;460:392–396. Available from: https://doi.org/10.1038/nature08221
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends lifespan of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. Available from: https://doi.org/10.1093/gerona/glq178
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, et al. Mechanisms of lifespan extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35-46. Available from: https://doi.org/10.1016/j.cmet.2009.11.002
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15(5):713-24. Available from: https://doi.org/10.1016/j.cmet.2012.04.004
- Dikicioglu D, Dereli Eke E, Eraslan S, Oliver SG, Kirdar B. Saccharomyces cerevisiae adapted to grow in the presence of low-dose rapamycin exhibit altered amino acid metabolism. Cell Commun Signal. 2018;16(1):85. Available from: https://doi.org/10.1186/s12964-018-0345-4
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, et al. If started early in life, metformin treatment increases lifespan and postpones tumors in female SHR mice. Aging (Albany NY). 2011;3(2):148-157. Available from: https://doi.org/10.18632/aging.100283
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. Available from: https://doi.org/10.1038/ncomms3192
- Yang Y, Lu X, Liu N, Ma S, Zhang Y, Jiang M, et al. Metformin decelerates aging clock in male monkeys. Cell. 2024; Available from: https://doi.org/10.1016/j.cell.2024.08.021
- Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of aging independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31-44. Available from: https://doi.org/10.1016/j.arr.2016.08.004
- Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Corrigendum: Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2017;545(7652):116. Available from: https://doi.org/10.1038/nature15766
- Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4). Available from: https://doi.org/10.1038/emm.2016.16
- Zhao L, Cao J, Hu K, He X, Yun D, Tong T, et al. Sirtuins and their biological relevance in aging and age-related diseases. Aging Dis. 2020;11(4):927-945. Available from: https://doi.org/10.14336/AD.2019.0820
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191-196. Available from: https://doi.org/10.1038/nature01960
- Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464-471. Available from: https://doi.org/10.1016/j.tcb.2014.05.001
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending lifespan. Cell Metab. 2008;8(2):157-68. Available from: https://doi.org/10.1016/j.cmet.2008.06.011
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528-36. Available from: https://doi.org/10.1016/j.cmet.2011.08.014
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7(1):78-88. Available from: https://doi.org/10.1111/j.1474-9726.2007.00355.x
- Yoshino J, Baur JA, Imai S. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018;27(3):513-528. Available from: https://doi.org/10.1016/j.cmet.2017.11.002
- Freeberg KA, Udovich CC, Martens CR, Seals DR, Craighead DH. Dietary supplementation with NAD+-boosting compounds in humans: current knowledge and future directions. J Gerontol A Biol Sci Med Sci. 2023;78(12):2435-2448. Available from: https://doi.org/10.1093/gerona/glad185
- Fonseca-Correa JI, Correa-Rotter R. Sodium-glucose cotransporter 2 inhibitors mechanisms of action: A review. Front Med (Lausanne). 2021;8:777861. Available from: https://doi.org/10.3389/fmed.2021.777861
- Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146:18:1383-1405. Available from: https://doi.org/10.1161/CIRCULATIONAHA.122.060283
- Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1730-5. Available from: https://doi.org/10.2337/dc15-0630
- Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. Available from: https://doi.org/10.2337/db15-1126
- Hoong CWS, Chua MWJ. SGLT2 inhibitors as calorie restriction mimetics: Insights on longevity pathways and age-related diseases. Endocrinology. 2021;162. Available from: https://doi.org/10.1210/endocr/bqab079
- Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. Available from: https://doi.org/10.2337/db15-1126
- Pietschner R, Kolwelter J, Bosch A, Striep K, Jung S, Kannenkeril D, et al. Effect of empagliflozin on ketone bodies in patients with stable chronic heart failure. Cardiovasc Diabetol. 2021;20:219. Available from: https://doi.org/10.1186/s12933-021-01410-7
- Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65(9):2784-94. Available from: https://doi.org/10.2337/db16-0694
- Packer M. Autophagy-dependent and -independent modulation of oxidative and organellar stress in the diabetic heart by glucose-lowering drugs. Cardiovasc Diabetol. 2020;19:62. Available from: https://doi.org/10.1186/s12933-020-01041-4
- Deng X, Zhang C, Wang P, Wei W, Shi X, Wang P, et al. Cardiovascular benefits of empagliflozin are associated with gut microbiota and plasma metabolites in type 2 diabetes. J Clin Endocrinol Metab. 2022;107(7):1888-1896. Available from: https://doi.org/10.1210/clinem/dgac210
- Afsar B, Afsar RE, Lentine KL. The impact of sodium-glucose cotransporter inhibitors on gut microbiota: a scoping review. J Diabetes Metab Disord. 2024;23:497-508. Available from: https://link.springer.com/article/10.1007/s40200-024-01435-1
- Kim MN, Moon JH, Cho YM. Sodium-glucose cotransporter-2 inhibition reduces cellular senescence in the diabetic kidney by promoting ketone body-induced NRF2 activation. Diabetes Obes Metab. 2021;23:2561-71. Available from: https://doi.org/10.1111/dom.14503
- Katsuumi G, Shimizu I, Suda M, Yoshida Y, Furihata T, Joki Y, et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat Aging. 2024;4:926–938. Available from: https://doi.org/10.1038/s43587-024-00642-y
- Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, et al. SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients. Hypertension. 2022;79(8):1633-1643. Available from: https://doi.org/10.1161/HYPERTENSIONAHA.122.18954
- Xu J, Kitada M, Ogura Y, Liu H, Koya D. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells. 2021 Jun 10;10(6):1457. Available from: https://doi.org/10.3390/cells10061457
- Packer M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat Rev Cardiol. 2023;20:443–462. Available from: https://www.nature.com/articles/s41569-022-00824-4
- Hoong CWS, Chua MWJ. SGLT2 inhibitors as calorie restriction mimetics: insights on longevity pathways and age-related diseases. Endocrinology. 2021;162(8). Available from: https://doi.org/10.1210/endocr/bqab079
- Hess DA, Terenzi DC, Verma S. Heal thyself: SGLT2 inhibition limits regenerative cell exhaustion and heals damaged vessels. Diabetes. 2021;70(8):1620–1622. Available from: https://doi.org/10.2337/db21-0574
- Moix S, Sadler MC, Kutalik Z, Auwerx C. Breaking down causes, consequences, and mediating effects of telomere length variation on human health. Genome Biol. 2024;25:125. Available from: https://doi.org/10.1186/s13059-024-03269-9
- Aguilera A, García-Muse T. Causes of genome instability. Annu Rev Genet. 2013;47:1-32. Available from: https://doi.org/10.1146/annurev-genet-111212-133232
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-28. Available from: https://doi.org/10.1056/NEJMoa1504720
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. Available from: https://doi.org/10.1056/NEJMoa1612971
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. Available from: https://doi.org/10.1056/NEJMoa1812389
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413-1424. Available from: https://doi.org/10.1056/NEJMoa2022190
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451-1461. Available from: https://doi.org/10.1056/NEJMoa2107036
- McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. Available from: https://doi.org/10.1056/NEJMoa1911303
- Wang Y, Gao T, Meng C, Li S, Bi L, Geng Y, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an updated systematic review and meta-analysis. Eur J Med Res. 2022;27(1):314. Available from: https://doi.org/10.1186/s40001-022-00642-2
- Lazzeroni D, Villatore A, Souryal G, Pili G, Peretto G. The aging heart: a molecular and clinical challenge. Int J Mol Sci. 2022;23:16033. Available from: https://doi.org/10.3390/ijms232416033
- Chiao YA, Rabinovitch PS. The aging heart. Cold Spring Harb Perspect Med. 2015;5(9). Available from: https://doi.org/10.1101/cshperspect.a025148
- Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. 2017;69(15):1952-1967. Available from: https://doi.org/10.1016/j.jacc.2016.12.002
- Li H, Hastings MH, Rhee J, Trager LE, Roh JD, Rosenzweig A. Targeting age-related pathways in heart failure. Circ Res. 2020;126:533–551. Available from: https://doi.org/10.1161/circresaha.119.315889
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, et al. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877-82. Available from: https://doi.org/10.1016/j.jamda.2013.05.009
- Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. Available from: https://doi.org/10.1016/j.cardiores.2004.12.026
- Desjardins J, Zhang Y, Thai K, Kabir G, Gilbert R, Connelly K. Empagliflozin reduces LV mass and improves diastolic function in an experimental model of heart failure with preserved EF. Can J Cardiol. 2022;33.
- Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. 2019;140(21):1693-1702. Available from: https://doi.org/10.1161/circulationaha.119.042375
- Novo G, Guarino T, Di Lisi D, Biagioli P, Carluccio E. Effects of SGLT2 inhibitors on cardiac structure and function. Heart Fail Rev. 2023;28(3):697-707. Available from: https://doi.org/10.1007/s10741-022-10256-4
- Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5(6):632-644. Available from: https://doi.org/10.1016/j.jacbts.2020.02.004
- Adam CA, Anghel R, Marcu DTM, Ovidiu Mitu O, Roca M, Mitu F. Impact of sodium-glucose cotransporter 2 (SGLT2) inhibitors on arterial stiffness and vascular aging—what do we know so far? (A narrative review). Life (Basel). 2022;12(6):803. Available from: https://doi.org/10.3390/life12060803
- Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17(4):302-307. Available from: https://doi.org/10.1053/j.ackd.2010.05.002
- Fang Y, Gong AY, Haller ST, Dworkin LD, Liu Z, Gong R. The ageing kidney: molecular mechanisms and clinical implications. Ageing Res Rev. 2020;63:101151. Available from: https://doi.org/10.1016/j.arr.2020.101151
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. Available from: https://doi.org/10.1056/nejmoa2024816
- The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117-127. Available from: https://doi.org/10.1056/nejmoa2204233
- Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomized clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691-704. Available from: https://doi.org/10.1016/s2213-8587(18)30141-4
- Kluger AY, Tecson KM, Lee AY, Lerma EV, Rangaswami J, Lepor NE, et al. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019;18(1):99. Available from: https://doi.org/10.1186/s12933-019-0903-4
- Xu XM, Cai GY, Bu R, Wang WJ, Bai XY, Sun XF, et al. Beneficial effects of caloric restriction on chronic kidney disease in rodent models: a meta-analysis and systematic review. PLoS One. 2015;10(12). Available from: https://doi.org/10.1371/journal.pone.0144442
- Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146(18):1383-1405. Available from: https://doi.org/10.1161/circulationaha.122.061732
- Au PCM, Tan KCB, Lam DCL, Cheung BWY, Wong ICK, Kwok WC, et al. Association of sodium-glucose cotransporter 2 inhibitor vs dipeptidyl peptidase-4 inhibitor use with risk of incident obstructive airway disease and exacerbation events among patients with type 2 diabetes in Hong Kong. JAMA Netw Open. 2023;6(1). Available from: https://doi.org/10.1001/jamanetworkopen.2022.51177
- Jeong HE, Park S, Noh Y, Bea S, Filton KB, Yu OHY, et al. Association of adverse respiratory events with sodium-glucose cotransporter 2 inhibitors versus dipeptidyl peptidase 4 inhibitors among patients with type 2 diabetes in South Korea: a nationwide cohort study. BMC Med. 2023;21:47. Available from: https://doi.org/10.1186/s12916-023-02765-2
- Hierro-Bujalance C, Infante-Garcia C, Marco A, Herrera M, Carranza-Naval MJ, Suarez J, et al. Empagliflozin reduces vascular damage and cognitive impairment in a mixed murine model of Alzheimer’s disease and type 2 diabetes. Alzheimers Res Ther. 2020;12:40. Available from: https://doi.org/10.1186/s13195-020-00607-4
- Siao WZ, Lin TK, Huang JY, Tsai CF, Jong GP. The association between sodium-glucose cotransporter 2 inhibitors and incident dementia: a nationwide population-based longitudinal cohort study. Diab Vasc Dis Res. 2022;19(3):14791641221098168. Available from: https://doi.org/10.1177/14791641221098168
- Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T, et al. SGLT-2 inhibitors in cancer treatment—mechanisms of action and emerging new perspectives. Cancers (Basel). 2022;14(23):5811. Available from: https://doi.org/10.3390/cancers14235811
- Benedetti R, Benincasa G, Glass K, Chianese U, Vietri MT, Congi R, et al. Effects of novel SGLT2 inhibitors on cancer incidence in hyperglycemic patients: a meta-analysis of randomized clinical trials. Pharmacol Res. 2022;175:106039. Available from: https://doi.org/10.1016/j.phrs.2021.106039
- Mori Y, Duru OK, Tuttle KR, Fukuma S, Taura D, Harada N, et al. Sodium-glucose cotransporter 2 inhibitors and new-onset type 2 diabetes in adults with prediabetes: systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2022;108(1):221-231. Available from: https://doi.org/10.1210/clinem/dgac591
- Cardoso R, Graffunder FP, Ternes CMP, Fernandes A, Rocha AV, Fernandes G, et al. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100933. Available from: https://doi.org/10.1016/j.eclinm.2021.100933
- Bhattarai M, Salih M, Regmi M, Al-Akchar M, Deshpande R, Niaz Z, et al. Association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in patients with type 2 diabetes and other risk factors for cardiovascular disease: a meta-analysis. JAMA Netw Open. 2022;5(1). Available from: https://doi.org/10.1001/jamanetworkopen.2021.42078
- Silverii GA, Monami M, Mannucci E. Sodium-glucose co-transporter-2 inhibitors and all-cause mortality: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2020;23:1052-1056. Available from: https://doi.org/10.1111/dom.14286
- Salah HM, Al'Aref SJ, Khan MS, Al-Hawwas M, Vallurupalli S, Mehta JL, et al. Effect of sodium-glucose cotransporter 2 inhibitors on cardiovascular and kidney outcomes–systematic review and meta-analysis of randomized placebo-controlled trials. Am Heart J. 2020;232:10-22. Available from: https://doi.org/10.1016/j.ahj.2020.10.064
- Chung MC, Hsu HT, Chang CH, Hung PH, Hsiao PJ, Wu LY, et al. Association of SGLT2 inhibitors with lower incidence of death in type 2 diabetes mellitus and causes of death analysis. Sci Rep. 2022;12:10147. Available from: https://doi.org/10.1038/s41598-022-13760-7
- Miller RA, Harrison DE, Allison DB, Bogue M, Debarba L, Diaz V, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. HERO. 2020;5. Available from: http://dx.doi.org/10.1172/jci.insight.140019
- Snyder JM, Casey KM, Galecki A, Harrison DE, Jayarathne H, Kumar N, et al. Canagliflozin retards age-related lesions in heart, kidney, liver, and adrenal gland in genetically heterogeneous male mice. GeroScience. 2022;44(6):1191-1204.
- Katsuumi G, Shimizu I, Suda M, Yoshida Y, Furihata T, Joki Y, et al. SGLT2 inhibition eliminates senescent cells and alleviates pathological aging. Nat Aging. 2024;4:926-938. Available from: https://www.nature.com/articles/s43587-024-00642-y